The European Patent Convention for Foreign Practitioners
Publication | 18.01.2019
Read the complete book here.
Transcript:
Table of Contents
PART A Substantive Requirements of European Patent Law
- Disclosure concepts and novelty
- Inventive Step
- Prior Art
- Sufficency of Disclosure
- Patentable Subject Matter I
- Patentable Subject Matter II
PART B Prosecution of European Patent Application
- Filing of EP Applications
- Claims
- Priority
- European Search
- Substantive Examination
- Amendments
- Remedies
PART C Procedures after Grant
PART D Prosecution of European Patent Applications
Introduction
PART A Substantive Requirements of European Patent Law
I. Disclosure concepts and novelty
- The Law
- Fundamental Concepts of Disclosure
- Novelty Assessment
- Novelty by Selection
- Novelty by Use Indication
- Special Cases
Introduction
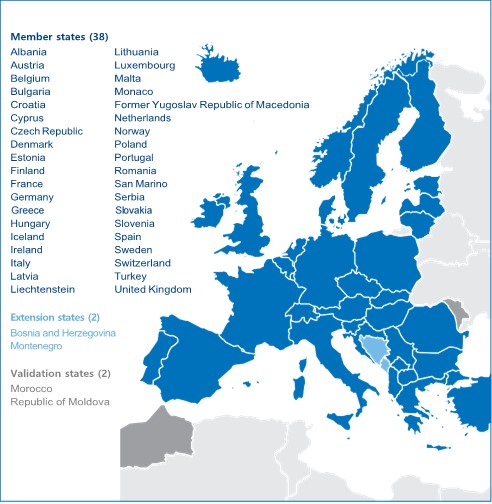
Patent law in Europe is complex in that national patent laws co-exist with the European Patent Convention (EPC). Since the EPC allows obtaining patent protection in 38 countries through a single procedure for the grant of patents, the EPC granting authority, the European Patent Office (EPO), has become by far the most important patent office in Europe. Currently, all the member states of the European Union together with Albania, Croatia, Macedonia, Iceland, Liechtenstein, Monaco, Norway, San Marino, Serbia, Switzerland and Turkey are members of the EPC.
PART A Substantive Requirements of European Patent Law
I. Disclosure Concepts and Novelty
1. The Law
The novelty requirement stems from Article 52 (1) EPC. This article reads as follows:
(1) European patents shall be granted for any inventions, in all fields of technology, provided that they are new, involve an inventive step and are susceptible of industrial application.
Hence, the novelty requirement is a fundamental requirement that must generally be fulfilled by all valid European patents at all stages of procedure. Novelty is defined in relation to the state of the art in subsection (1) of Article 54 EPC:
(1) An invention shall be considered to be new if it does not form part of the state of the art.
The above definition is noteworthy in so far as it is a negative definition. This means that there is an initial presumption that the invention of interest is novel unless there is specific state of the art that anticipates the invention. It is a direct consequence of this negative definition that the burden of proving lack of novelty initially rests on the examiner or opponent. The examiner is not entitled to assert lack of novelty without citing a specific piece of the state of the art that allegedly anticipates the invention of interest.
The state of the art is defined in the following subsections (2) and (3) of Article 54 EPC. While subsection (2) defines the normal state of the art, subsection (3) defines that earlier EP applications constitute hypothetical prior art for the assessment of novelty only even if they are post-published:
(2) The state of the art shall be held to comprise everything made available to the public by means of a written or oral description, by use, or in any other way, before the date of filing of the European patent application.
(3) Additionally, the content of European patent applications as filed, the dates of filing of which are prior to the date referred to in paragraph 2 and which were published on or after that date, shall be considered as comprised in the state of the art.
Regarding subsection (2), it should be considered that the listed types of disclosure are merely provided as examples since the wording “in any other way” covers any conceivable form of making the relevant information available to the public. For instance, internet disclosures may also form relevant state of the art. However, a stricter standard of proof compared to traditional documents is usually applied (Guidelines G-IV 7.5). Neither the meaning of “public” nor the standard for determining whether relevant information has indeed been “made available” are defined by law. The applicable standards established by case law are discussed below.
While subsection (2) expressly refers to the filing date of the European patent application, it should be kept in mind that a valid priority claim has the effect that this date is replaced by the priority date (Art. 89 EPC).
Two specific scenarios concerning medical inventions are dealt with in subsections (4) and (5) of Article 54 EPC. These subsections are intended to provide compensation to the pharmaceutical industry for the prohibition of obtaining patent protection for methods of treatment and the like in Article 53
(c) EPC. Subsection (4) introduces the so-called “first medical use” claim, whereas subsection (5) provides a legal basis for claiming second and further medical uses:
(4) Paragraphs 2 and 3 shall not exclude the patentability of any substance or composition, comprised in the state of the art, for use in a method referred to in Article 53(c), provided that its use for any such method is not comprised in the state of the art.
(5) Paragraphs 2 and 3 shall also not exclude the patentability of any substance or composition referred to in paragraph 4 for any specific use in a method referred to in Article 53(c), provided that such use is not comprised in the state of the art.
Subsections 4 and 5 expressly refer to the patentability of any “substance or composition”. Current case law interprets this wording as restricting the applicability of these subsections to inventions involving an active substance that undergoes a molecular interaction with a target substance (T 2003/08). Medical devices that are used in treatment methods based on purely mechanical interaction are thus not susceptible to protection by 1st or 2nd medical use claims (T 1069/11).
2. Fundamental Concepts of Disclosure
2.1 Public
The concept of “public” is applied in a broad manner in the case law of the EPO. Even a single person who is not bound by confidentiality and who can therefore freely pass on the information to other people will be regarded as “public”. However, if the information is conveyed by oral disclosure (e.g. in a lecture), it is necessary that at least one member of the audience has sufficient skills to understand the information in order to be able to pass it on to others (T 877/90). Regarding disclosures via the internet, a test for public availability of a document stored on the World Wide Web and accessible via a specific URL was developed in decision T 1553/06. In summary, it was considered decisive in this decision whether the document could be found with the help of a public web search engine by using one or more keywords all related to the essence of the content of that document, and whether the document remained accessible at that URL for a period of time long enough for a member of the public to have direct and unambiguous access to it. For e-mails transmitted via the internet, the principle of confidentiality is deemed to apply, and thus they are not considered to be publicly available. The possibility of accessing the content of an e-mail by hacking does not change that principle, as long as an actual disclosure of the information contained in the e-mail in breach of the confidentiality has not been proven. Therefore, the content of an e-mail does not become available to the public for the sole reason that it was transmitted via the internet (T 2/09).
2.2 Direct and Unambiguous Disclosure
When assessing the disclosure of a prior art document, the EPO applies a strict formalistic approach, according to which information is only disclosed, which the skilled person would derive directly and unambiguously from the document in question using the common general knowledge at the time of its publication (G 2/98, G 2/10 and T 1363/12).
2.3 Whole Content Approach and Combinations of Disclosure
The whole content of the prior art document is to be considered for the novelty assessment, including definitions of technical terms provided in other parts of the same document. Even information provided in other “secondary” documents may be included in said disclosure of a “primary”document if there is a sufficiently clear cross-reference in the primary document to said information in the secondary document (Guidelines G-IV 8).
However, for raising a novelty objection, it is not possible to combine pieces of information that are disclosed in the same document but in a different technical context (Guidelines G-VI 1, T 305/87). Similarly, information provided in different documents must not be combined in the absence of a sufficiently clear cross- reference (Guidelines G-VI 1).
2.4 Enabling Disclosure
The disclosure of a prior art document is considered to be relevant state of the art only if said disclosure enables the skilled person to reproduce this disclosure (Guidelines G-IV 2 and G-VI 4). For instance, if a chemical compound mentioned in the prior art could not be produced since the starting materials and intermediates were unavailable, the compound does not belong to the relevant state of the art (T 206/83).
2.5 Implicit Disclosure
The disclosure of a prior art document is not restricted to what is expressly mentioned in said document. In addition to the express disclosure of a prior art document, the EPO also takes into account further information implied by said express disclosure. The concept of “implicit disclosure” encompasses all further information that is not expressly mentioned but follows as an inevitable consequence of the expressly mentioned prior art disclosure (Guidelines G-VI 6). When assessing implicit disclosures, the EPO applies a strict approach according to which it must be beyond all reasonable doubt that the information of interest is an inevitable consequence of the express teaching of the prior art document (T 793/93).
2.6 Disclosure by Public Prior Use
In cases of public prior use and in cases of implicit disclosure, the question sometimes arises as to which additional information of a product has been made available ifthe product itself was publicly available. The EPO distinguishes between “intrinsic properties” and “extrinsic properties”. Intrinsic properties are generally considered to be made available by a publicly available product irrespective of whether or not the skilled person would have had a motivation to analyze the product and/or whether the analysis would involve a significant effort (G 1/92). Intrinsic properties are product characteristics, which are inherent to the product of interest and not linked to external testing conditions. A typical example would be the chemical structure of a small organic molecule. Extrinsic properties, on the other hand, are generally not considered to be disclosed by a public availability of the product. These are properties that are exhibited by the product of interest only if it is subjected to specific test conditions. A typical example would be the suitability of a small organic molecule for treating a particular disease.
2.7 Degree of Individualization
When assessing prior art disclosures, the EPO also considers the degree of individualization of the prior art disclosure and compares it to that of the claim of interest. A disclosure of a higher degree of individualization always takes away novelty of a claim, wherein the corresponding feature is specified at a lower degree of individualization. Conversely, novelty may or may not be established if the claim of interest characterizes a feature at a higher degree of individualization as compared to the degree of individualization of the corresponding prior art disclosure. For instance, the individualized disclosure of “copper” anticipates the more generic concept “metal”. Conversely, the disclosure of the generic concept “metal” in the prior art does not anticipate a later claim with the individualized feature “copper” (Guidelines G-VI 5). The applicable approaches for assessing novelty by selection are explained in greater detail below.
3. Novelty Assessment
The assessment of novelty in proceedings before the EPO is usually carried out on a feature-by-feature basis. Novelty is normally denied only if it is established that the prior art document contains a direct and unambiguous disclosure of each and every feature in the claim. There are, however, some exceptions before the EPO, mainly due to specific rules concerning the burden of proof. Exceptional situations are for instance as follows:
- Novelty objections can be raised against claims with parameter ranges if the parameter is an unusual parameter and if the prior art document does not provide any information on said parameter, provided that all other features of the claim are disclosed in this document. The burden of proving that the parameter features is not fulfilled by the prior art disclosure rests with applicant (Guidelines G-VI 6).
- Novelty objections can also be raised against claims with product-by- process features even if the prior art document does not mention the specified process. This is because product-by-process claims are construed at the EPO to protect the product per se (T 150/82) so that a product produced by a different process is also covered, provided the characteristics of the products are indistinguishable. The burden of proving that the specified process imparts distinct product characteristics that are not found in the prior art product rests with applicant (Guidelines F-IV 4.12).
- Special rules also apply for use indications. These are discussed in section 5 below.
4. Novelty by Selection
4.1 General Considerations
The EPO acknowledges the concept of “novelty by selection”. Depending on the factual scenario, different approaches have been developed by the EPO for assessing novelty of different types of selections. However, irrespective of the type of selection, the general rule applies that a novel selection is possible only if the prior art document does not contain an individualized disclosure, such as a specific example, falling within the selected area.
4.2 Selection from Generic Term
As noted above, a novel selection can be made from a general term or another type of general disclosure. In such a situation novelty is usually acknowledged in the absence of a more specific (individualized) disclosure in the prior art document.
4.3 Selection from List
If a selection is made among alternatives that are disclosed in individualized form as members of a list in the prior art document, novelty is generally denied if said selection involves merely the selection of an individual feature from a single list. Conversely, if the claimed subject-matter can be derived from the prior art document only by means of a two-fold selection from two lists of some length, novelty is usually acknowledged (T 12/81, T 7/86, Guidelines G-VI 8(i)). The same conclusion applies also in cases where three or more selections from a corresponding number of lists are involved. This concept is sometimes referred to as the “two-lists principle”. It applies if the individual selections involve a selection from a list of individualized alternatives (e.g., alternative substituent definitions in a Markush formula). The two-lists principle also applies if one or more of the individual selections involves another type of selection such as the selection of a numerical sub-range from a broader known numerical range.
4.4 Selection from Numerical Range
If there is a single selection of a sub-range from a broader known range, stricter rules apply. Usually, novelty is acknowledged in this situation only if the following 3 criteria are fulfilled (T 198/84, T 279/89 and Guidelines G-VI 8(ii)):
(a) the selected sub-range is narrow compared to the known range;
(b) the selected sub-range is sufficiently far removed from any specific examples, preferred sub-ranges or end-points of the known range;
(c) the selected range is not an arbitrary specimen of the prior art, i.e. not a mere embodiment of the prior art, but another invention (purposive selection, new technical teaching).
There has been some controversy in recent EPO case law whether the last criterion (c) is appropriate for the assessment of novelty. Some decisions (T 1233/05, T 230/07 and T 1130/09) held that said last criterion (c) is appropriate for the assessment of inventive step, but should not be applied for the assessment of novelty. Others (T 1827/08 and T 126/09) rejected this view. It thus remains to be seen whether discounting criterion (c) is a general trend. However, even the most recent version of the Guidelines still mentions criterion (c) as an essential precondition for novelty.
4.5 Partial Overlap with Known Numerical Range
Different criteria may apply in cases where a numerical range is selected from a known range such that the selected range partially overlaps with the known range. In this situation, it is frequently assessed whether the skilled person studying the prior art document would “seriously contemplate” working in the area of overlap (T 26/85 and Guidelines G-VI 8(iii)).
4.6 Selection of Sub-genus from Generically Defined Area
When assessing prior art disclosures, the EPO also considers the degree of individualization of the prior art disclosure and compares it to that of the claim of interest. A disclosure of a higher degree of individualization always takes away novelty of a claim, wherein the corresponding feature is specified at a lower degree of individualization. Conversely, novelty may or may not be established if the claim of interest characterizes a feature at a higher degree of individualization as compared to the degree of individualization of the corresponding prior art disclosure. For instance, the individualized disclosure of “copper” anticipates the more generic concept “metal”. Conversely, the disclosure of the generic concept “metal” in the prior art does not anticipate a later claim with the individualized feature “copper” (Guidelines G-VI 5). The applicable approaches for assessing novelty by selection are explained in greater detail below.
4.7 Selection of Purity Level
The characterization of a particular purity level in a small organic molecule normally does not permit to establish novelty by selection even if the prior art disclosure does not contain any information on the obtained purity level. Generally, the disclosure of a small organic compound is understood as the disclosure of said compound in any degree of purity that is available by applying conventional purification methods (T 990/96). Novelty is acknowledged only in special situations such as the following ones:
- At the priority date conventional purification means did not allow f the specified purity level (T 728/98);
- the claim is directed to a manufacturing process and the purity level characterizes the starting material rather than the end product (T 786/00);
- the claim is directed to a composition and the specified purity level characterizes an individual component of said composition (T 112/00), although this depends on the technical details (T 803/01); and
- the claim is directed to a high molecular weight compound and the purity indication is a purposive selection essential for accomplishing the inventive technical effects and there was no desirability in the relevant prior art to obtain the claimed grade of purity (T 142/06).
5. Novelty by Use Indication
5.1 Use Indication in Non-Medical Product Claim
A typical claim wording would be “Product X for (non-medical) use Y”. The EPO treats use indications differently depending on the type of claim that is involved. If the claim of interest is a product claim in technical fields other than medicine, use indications are merely considered as specifying that the claimed product must be suitable for the indicated purpose (Guidelines F-IV 4.13). This means that novelty can be denied even if the intended use is not mentioned in the cited prior art document, provided that suitability for the indicated use is given for the prior art product.
5.2 Use Indication in Medical Product Claim
When assessing prior art disclosures, the EPO also considers the degree of individualization of the prior art disclosure and compares it to that of the claim of interest. A disclosure of a higher degree of individualization always takes away novelty of a claim, wherein the corresponding feature is specified at a lower degree of individualization. Conversely, novelty may or may not be established if the claim of interest characterizes a feature at a higher degree of individualization as compared to the degree of individualization of the corresponding prior art disclosure. For instance, the individualized disclosure of “copper” anticipates the more generic concept “metal”. Conversely, the disclosure of the generic concept “metal” in the prior art does not anticipate a later claim with the individualized feature “copper” (Guidelines G-VI 5). The applicable approaches for assessing novelty by selection are explained in greater detail below.
5.3 Medical Use-type Claims
Claims directed to medical uses as such (“Use of compound X for treating disease Y”) are considered to be equivalent to claims directed to methods of treatment and they are therefore prohibited under Article 53(c) EPC. Claims drafted in the so-called “Swiss-type” (“Use of compound X for manufacturing a medicament for treating disease Y”) were introduced into patent practice of the EPO by the Enlarged Board of Appeal as a possibility for protecting 2nd and further medical uses before EPC 2000 came into force (G 1/83, G 5/83, and G 6/83). “Swiss-type” claims, however, are no longer available for protecting second and further medical uses since they are no longer considered necessary in view of Article 54 (5) EPC, which was introduced into the EPC 2000. An exception is made only for patents and patent applications having an effective date earlier than January 29, 2011 (G 2/08). Even for such earlier patents and patent applications, the simultaneous presence of use-restricted product claims and “Swiss-type” claims is not generally allowed in the same application (T 1570/09) but may be allowed in a parent application/divisional application situation (T 1780/12).
5.4 Non-medical Use-type Claims
Non-medical use claims (“Use of product X for (non-medical) purpose Y”) allow makingtheuseindicationaneffectivedistinguishingfeature. Hence, ifthespecified use is not disclosed in the prior art document, novelty should be acknowledged. It is even possible to establish novelty by specifying a use directed to achieving a previously unknown technical effect if the practical implementation of the new use does not differ from the practical implementation of the known use (G 2/88 and G 6/88). This seemingly surprising view can be rationalized by considering that the new effect may provide the skilled person with new fields of application of the known use. Moreover, it may be kept in mind that the new effect is normally an extrinsic property, which is not made available by the known prior art use (see section 2.6 above).
A distinction must be made if there is a use indication in a process claim. If the new use characterizes the process itself (e.g. regarding the field of application), it must be regarded as a functional process feature, which is effective in distinguishing the claimed process from the known prior art process (T 848/93). Similarly, a new use is also effective in establishing novelty if the claim is directed to a process involving the use of a known substance and said new use pertains to the accomplishment of a newly discovered effect of said known substance (T 304/08). Conversely, if the process claim is directed to the manufacture of a product and the new use merely characterizes the product obtained by the specified process, it will not be effective in establishing novelty even if the prior art disclosure does not mention the obtained product characteristic (T 210/93).
6. Special cases
6.1 Negative Features
Negative features may be used to establish novelty by expressly excluding certain embodiments from the claim scope, which are known from the prior art. Such negative features can be based on corresponding negative disclosures in the original application text (i.e. indications that the relevant embodiments are less desirable), they can be based on positive disclosures in the original application text (i.e. indications that the relevant embodiments are part of the disclosed invention) and, in some instances, they may be formulated without any basis in the original application (so-called “undisclosed disclaimers”).
Negative features based on corresponding negative original disclosures are unproblematic.
Negative features based on positive original disclosures (so-called “disclosed disclaimers”) may be allowed, provided that the exclusion of certain embodiments from the claim scope does not lead to the situation that the subject-matter remaining in the claim after the introduction of the disclaimer is not, be it explicitly or implicitly, directly and unambiguously disclosed to the skilled person using common general knowledge, in the application as filed (G 2/10). This means that the exclusion of individual examples should normally be allowed similar to the exclusion of individual features that are originally disclosed as specific alternatives in the form of a list among other alternatives. Added matter issues may arise if a disclosed disclaimer is formulated, wherein two or more features are isolated from the original disclosure and combined with each other to characterize the embodiments to be excised from the claim scope.
The formulation of undisclosed disclaimers is possible only in special circumstances, namely if one of the following situations is given (G 1/03):
- The disclaimer is used for establishing novelty over a post-published prior art reference under Article 54 (3) EPC.
- The disclaimer is used for establishing novelty over an accidental anticipation. Thereby, the notion of “accidental anticipation” is construed narrowly and means a prior art disclosure that is so unrelated to and remote from the claimed invention that the person skilled in the art would never have taken it into consideration when making the invention. In such instances, disclaimers are available even if the accidental disclosure is pre-published prior art under Article 54 (2) EPC.
- The disclaimer is used for excluding subject-matter from the claim scope, which is excluded from patentability for non-technical reasons. For example, methods of treatment of the animal or human body might be qualified as “non-therapeutic” to avoid the prohibition of Article 53(c) EPC.
In all of the above situations, the disclaimer must fulfill the clarity requirement and it must not be relevant for the assessment of inventive step. Moreover, the disclaimer must not excise more than is necessary for establishing novelty unless a broader formulation of the disclaimer is appropriate in view of the clarity requirement (T 2130/11).
6.2 Unclear Features
It is normally difficult or even impossible to establish novelty on the basis of unclear features. Before grant, the examiner will normally raise objections of lack of novelty and, additionally, lack of clarity (Guidelines F-IV 4.6). Both objections will need to be overcome independently of each other. While an unclear feature, for instance a relative term, might stay in the claim when there is no basis in the disclosure for a clear definition, it cannot be used by the applicant to distinguish his invention from the prior art, i.e. to establish novelty (Guidelines F-IV 4.6). After grant, the opposition division will normally interpret the claim in the broadest technically meaningful manner (T 686/96 and T 79/96). If this broadest technically meaningful interpretation encompasses prior art disclosures, novelty will be denied.
6.3 Parameter Reges and Error Margins
Problems may also arise if the claim contains a feature characterizing a numerical range and if the corresponding prior art parameter value is outside the numerical range but very close to one of the limiting values of the claimed range since, as a rule, “technical equality” will be understood as having the meaning of “identity within inevitable measurement errors or manufacturing tolerances” (T 605/97). Hence, it may become necessary to specify the claimed range more narrowly such that there is no overlap even when taking measurement errors into account (Guidelines G-VI 8.1).
6.4 Non-technical Features
Usually, for inventions that are partly or completely non-technical (e.g. business methods), the most critical obstacles are the general exclusion of non-technical subject-matter from patentability under Article 52 EPC, as well as the lack of contribution to inventive step under Article 56 EPC by non-technical features. In addition, non-technical features, to the extent that they do not interact with technical features to produce a technical effect, are also not effective for distinguishing a claimed invention from the cited prior art (T 154/04).
II. Inventive Step
- Introduction
- Legals Basis for Inventive Step
- State of the Art
- Person Skilled in the Art
- Not obvious?
- Problem-Solution-Approach
- Problem Inventions
- Summary
1. Introduction
To be awarded a patent it is generally accepted that the underlying invention must be something more than a trivial modification of that which was already known. Each jurisdiction legislates for this in its own way but the fundamental approach is essentially the same: the invention in question must not be obvious to somebody who is presumed to have ordinary skill in the art. A number of questions naturally arise from this approach. Who is this person of ordinary skill? What information is available to them? And how exactly does one determine if the invention in question would not have been obvious to that person?
Courts in the various jurisdictions around the world have grappled with these and similar questions and over time have developed their own ways to answer them. In this chapter we will look at how the Boards of Appeal at the EPO deal with this issue.
2. Legal Basis for Inventive Step
The EPC requires the invention to “involve an inventive step” (Art. 52(1) EPC). This requirement is considered to be fulfilled if the invention is “not obvious” to the person skilled in the art having regard to the state of the art (Art. 56 EPC).
Since being “not obvious” leads to the recognition that an invention involves an “inventive step”, these and related terms are oftentimes used interchangeably in European patent practice. This may lead to some confusion, particularly for those who are perhaps more accustomed to dealing with the US requirement of “non- obvious” (35 U.S.C. §103). In proceedings before the EPO, it might be helpful to think of the expression “involve an inventive step” as one of the legal tests for patentability and the state of being “not obvious” as the way to pass that test. However, before assessing if an invention is “not obvious” we must first establish what is meant by the expressions “state of the art” and “person skilled in the art”.
3. State of the Art
The state of the art is the body of public knowledge that the “person skilled in the art” is presumed to have access to when looking to solve technical problems. It is also the body of public knowledge from which a “closest prior art” will be chosen when performing the problem-solution approach (discussed in more detail later). Therefore, correctly establishing the state of the art is a key component before evaluating if an invention is “not obvious”.
The state of the art comprises everything made available to the public (whether that is by written or oral description, by use, or by any other way) before the date of filing of the European patent application. If the application originates from a PCT application (EP-PCT), the filing date of the PCT application is decisive. Similarly, if a valid priority claim exists, then the filing date of the priority application is decisive. The so-called Art. 54(3) prior art is that which has not been made available to the public before the filing date and thus is not considered prior art for the purpose of assessing inventive step under Art. 56 EPC. A public disclosure of the invention due to an abuse of some kind (e.g. a breach of confidence) is also not part of the state of the art provided that the EP or EP-PCT application (not the priority application!) is filed within six months of that disclosure (Art. 55 EPC).
4. Person Skilled in the Art
The person skilled in the art – or the “skilled person” for short – plays a central role in determining if an invention involves an inventive step. After all, it is the skilled person’s knowledge and capability that is taken into account when assessing if an invention is “not obvious”.
Essentially, the skilled person is a hypothetical, experienced practitioner in the field where the invention solves a technical problem. He (or, once and for all, she) possesses no inventive capability but has at his disposal the means and capacity for normal routine work and experimentation. The skilled person is aware of the common general knowledge in the field which is usually established by disclosures in basic textbooks, handbooks, dictionaries, and the like. The skilled person is also presumed to have access to everything in the state of the art irrespective of its language, location, or time of disclosure. It is important to recognise that while access to the state of the art is absolute, this does not mean that the skilled person is familiar with everything in it, or that he will consider everything in it when looking to solve a problem. Rather, the skilled person will look to his own and neighbouring technical fields for solutions to any problems that he encounters. If the situation warrants, he may also look to more distant technical fields but only if he is prompted to do so. In cases where the solution to a problem spans multiple technical fields, the skilled person can be considered to be a group made up of a skilled person from each of the respective fields.
5. Not obvious?
While it is comparatively straightforward to establish the state of the art and the appropriate skilled person, the next step of assessing if an invention is “not obvious”is notan easy task. In fact, one mightsay thatitis impossible toconclusively establish that an invention is not obvious because there is always a possibility that some, as-yet, unconsidered prior art exists that renders the invention obvious.
In practice, rather than try to establish that an invention is not obvious, the burden initially rests with an examiner or an opponent to demonstrate that the Invention is obvious. If this burden cannot be discharged then the invention is presumed not to be obvious in view of the available evidence.
As to what is meant by “obvious”, the Boards of Appeal consistently refer to it as that which does not go beyond the normal progress of technology but merely follows plainly or logically from the prior art. In other words, something is obvious if it does not involve the exercise of any skill or ability beyond that which can be expected of the skilled person. This raises certain questions. For example, what starting point should be used when assessing if an invention is obvious? How much credence can we give to the inventor’s own view of his invention and its merits when making that assessment? It was to address issues such as these that the Boards of Appeal developed the so-called “problem-solution approach” (or the “PSA” for short) which, although not coded in the law, is nevertheless the standard approach that is used at the EPO for assessing if an invention is obvious.
6. Problem-Solution-Approach
Since an applicant generally discloses his invention in the context of a problem that he perceives to exist in the prior art, the applicant’s problem and the proposed solution must be regarded from the outset as being subjective in nature. The purpose of the PSA is to provide an objective assessment if an invention is obvious having regard to the state of the art. The PSA consists of three main stages which are usually set out as shown below:
1) determine the “closest prior art”;
2) establish the “objective technical problem” to be solved; and
3) consider whether or not the claimed invention, starting from the closest prior art and the objective technical problem, would have been obvious to the skilled person.
Essentially, in stage 1 what is sometimes called “the most promising springboard” to the invention is determined. In stage 2 the technical contribution made by the invention in view of this closest prior art is established. Finally, in stage 3 one considers if it would have been obvious to the skilled person that this technical contribution could be achieved by something falling within the scope of the claim in question.
6.1 Stage 1: The Closest Prior Art
The choice of closest prior art is perhaps the most crucial aspect of the PSA since everything else flows from this. Not only does the choice of closest prior art directly affect how the “objective technical problem” in stage 2 is formulated, it is starting from this prior art in stage 3 that one considers if the invention is obvious. The choice of closest prior art is often the single most important factor leading to an invention being considered obvious at the EPO. Therefore, it will not surprise the reader to learn that in proceedings before the EPO, particularly in opposition proceedings, a great deal of time and effort is often spent debating whether a particular disclosure qualifies as the closest prior art.
6.1.1 Conceived for the Same Purpose
To qualify as the closest prior art, the first consideration is that the disclosure must be something conceived for the same purpose as the invention because otherwise it cannot lead the skilled person in an obvious way to the invention. The following example illustrates this point.
Imagine a claim to a squash ball having a blue colour. The description of the application (or patent) explains that the blue colour reduces visible markings on the white walls of a squash court. Furthermore, it is also explained that the blue colour allows a player to more easily track the ball against the white wall of the squash court as compared to a ball of any other colour. Now imagine different prior art disclosures: (i) a black squash ball that was widely used for over one hundred years; (ii) a more recently developed green squash ball that is said to leave less visible markings on the walls of a squash court; and (iii) a golf ball that is coloured blue for purely aesthetic reasons. Which of these prior art disclosures represents a suitable starting point for a development that will lead in an obvious way to the claimed invention?
The golf ball undoubtedly contains the core feature upon which the invention is based (i.e. the blue colour). However, it is not a realistic starting point for any development leading in an obvious way to the invention. Without hindsight of the invention, any development of a golf ball will lead to something that is still a golf ball. It is only with hindsight that one would argue that the skilled person, looking to make a squash ball, would start from a golf ball. This kind of hindsight is largely avoided by considering only those disclosures that are conceived for the same purpose as the invention in question. In this case the black squash ball or the green squash ball is objectively a better starting point and therefore a more suitable choice as the closest prior art.
6.1.2 Similar Technical Problem
When assessing if an invention is obvious it is reasonable to give some weight to the problem faced by the inventor when he conceived his invention. Therefore, another important consideration when choosing the closest prior art is whether it aims to solve the same or a similar technical problem which the application (or patent) addresses. Prior art disclosures that aim to solve the same or a similar problem will be considered more promising starting points than prior art disclosures which do not address the problem at all. This does not mean to say that the closest prior art must be something that was known to the inventor when he conceived his invention. An inventor cannot be expected to have complete knowledge of the state of the art and it might very well be that the problem faced by the inventor is best tackled (or indeed has been solved) from a starting point of which he was not aware. In the example above, the green squash ball addresses at least one of the problems mentioned in the application (less visible markings on the wall) and so arguably could be considered a more suitable choice as the closest prior art than the black squash ball.
6.1.3 Most Relevant Technical Features in Common
When faced with a number of prior art disclosures that address the same or a similar technical problem as the invention, a further approach that is widely adopted at the EPO is to choose the prior art that has the most relevant technical features in common with the invention. In other words, the closest prior art is that single piece of prior art which addresses the same or a similar technical problem as the invention and which requires the minimum of structural modifications to reach the invention.
This approach is often adopted by examiners at the EPO for its practicality. After all, if the invention is not obvious from this single closest prior art then the examiner can assume (whether rightly or wrongly) that he is unlikely to find the invention obvious from a disclosure that shares even less features in common with the invention.
6.1.4 More than one closest prior art?
Two or more prior art disclosures are considered equally suited as the closest prior art if they address the same problem as the invention and each shares the same number of relevant technical features with the invention. In this situation it is widely accepted that stages 2 and 3 of the PSA should be performed for each of the disclosures in turn. Inventive step is then denied if the invention is found to be obvious starting from either disclosure.
Lengthy debates often occur if there are two or more prior art disclosures addressing the same problem as the invention where one leads in an obvious way to the invention but the other does not. Proponents of the “most relevant features in common”principle will argue that the closest prior art must be that which requires the minimum of structural modifications to reach the invention irrespective of the fact that it may not lead to the invention in an obvious way. This can lead to the strange scenario whereby the invention might have been considered obvious but for the discovery of that closest prior art. This is not a problem per se if one accepts as a matter of fact that the PSA must only be performed in view of a piece of prior art that is objectively chosen by some pre-defined criteria. However, it does seem to clash with the purpose of the exercise which is to determine, as best as possible, if the invention would have been obvious in the real world. Perhaps this Approach places too much emphasis on the literal meaning of the term “closest” in stage 1 of the PSA and not enough emphasis on the wording of the law itself which requires that one have regard to “the state of the art” (not just one or two pieces of it) when assessing if an invention is obvious.
Having said that, a number of decisions from the Boards of Appeal have accepted that if the skilled person has a choice of several workable routes which might lead to the invention, the rationale of the PSA requires that the invention be assessed relative to each of the workable routes.
Back to the table of contents
6.2 Stage 2: The Objective Technical Problem
After a suitable closest prior art disclosure has been identified, the next stage of the PSA is to establish what technical contribution the invention makes in view of that disclosure. Once established, the contribution is formulated as the so-called “objective technical problem” – that is, the problem that the skilled person must attempt to solve by adapting or modifying the closest prior art. Since the technical contribution will depend on the choice of the closest prior art, the objective technical problem may not necessarily correspond to the perceived or subjective problem that the inventor initially considered his invention to solve.
Stage 2 of the PSA is generally performed in a number of discrete steps as outlined below.
6.2.1 Distinguishing Technical Features
First, the invention is compared with the closest prior art to identify the distinguishing technical features – namely, those technical features of the invention that are not present in the closest prior art. For the purpose of this exercise, features that qualify as technical features are those that contribute to the technical character of the invention. This includes features which, if taken in isolation, might not reveal a technical aspect but nevertheless contribute to the overall technical character by interacting with other features of the invention to solve a technical problem (e.g. a computer program; a mathematical method; the colour blue in the example of the squash ball above, etc.). Naturally, the invention must contain at least one distinguishing technical feature otherwise it will already be deemed to have lacked novelty thus negating the need to consider inventive step at all.
6.2.2 Establishing a Technical Effect
Once the distinguishing technical features have been identified, a determination is made as to what technical effect, if any, can be attributed to those features and thus reflected in the objective technical problem to be solved. First and foremost, any technical effect that is to be relied upon must be something that the skilled person can derive from the application as filed. A technical effect might be self-evident by how the distinguishing technical feature interacts with the rest of the invention, with or without further explanation in the description (as is more common in mechanical cases). Sometimes the technical effect requires a more concrete disclosure (as is more common in the fields of chemistry and biotechnology). For example, it is probably not self-evident that it is easier for the human eye to track a blue squash ball in flight than a black or green squash ball. Accordingly, this effect should be mentioned in the application as filed or at least be derivable therefrom if the effect is to be relied upon when formulating the objective technical problem.
Secondly, the skilled person must find it at least plausible from the application as filed that the effect to be relied upon can be achieved by the invention (e.g. from an explanation of how the invention works, from data in the application, using his common general knowledge having regard to the disclosure in the application, etc.). In cases where the technical effect is recited as something to be achieved in the claim language itself (e.g. in a second medical use claim), this plausibility requirement is usually discussed under the mantle of sufficiency of disclosure.
The requirement that a technical effect is plausible does not mean that the application as filed must conclusively show that the effect is indeed achieved, or that the effect is something above and beyond that which is achieved by the closest prior art. If necessary, this may be demonstrated later with supplemental evidence (sometimes called “post-published evidence”). Supplementary evidence is most often required when the choice of closest prior art places the invention in a perspective that is different to that which was originally envisioned. This evidence, so long as it is timely filed and is not the sole basis for demonstrating plausibility of the purported technical effect, may be relied upon during prosecution, opposition and appeal proceedings at the EPO.
Finally, a technical effect needs to be established across the whole scope of the claim if it is to be taken into account when formulating the objective technical problem. In other words, all variants of the invention as claimed should be able to provide the effect. Objections that an effect has not been established across the whole scope of the claim are sometimes referred to as “AgrEvo” objections, named after the appeal case that first decided the issue. These objections typically occur in the field of chemistry and biotechnology where it is common practice to claim a myriad of different variants (e.g. using Markush formulae) but provide experimental evidence for only a handful of those variants. If the extent of the effect is in doubt, and the applicant wishes to rely on the technical effect when formulating the objective technical problem, then there are basically two options available: (i) file supplementary evidence that establishes the effect across the whole scope of the claim, or (ii) limit the claim to those variants where the technical effect has been established.
6.2.3 Formulating the Objective Technical Problem
The final part of stage 2 of the PSA is to explicitly formulate the objective technical problem to be solved. Essentially, the skilled person is tasked with modifying or adapting the closest prior art to provide the technical effect that the invention achieves over the closest prior art. Care should be taken at this stage not to include any pointers to the technical solution in the objective technical problem. For instance, in the squash ball example from above, a suitable objective technical problem might be to modify the green (or black) squash ball so that it can be tracked by the human eye more easily in flight. An inappropriately formulated problem would be, for example, to modify the colour of the black or green squash ball so that it can be tracked more easily in flight since this would then already contain a pointer to the solution (i.e. modify the colour).
If no technical effect has been demonstrated over the closest prior art, or if an effect has not been demonstrated over the whole scope of the claim, the objective technical problemtobe solved is toprovide an alternativetothatwhichwas already known from the closest prior art. The fact that the objective technical problem is to provide an alternative does not mean that a patent cannot be granted since it is not a requirement to show an improvement over the closest prior art. Alternative ways to solve problems that have already been solved are perfectly acceptable, provided of course that the proposed alternative solution is not obvious.
If a claim contains a mix of technical and non-technical features, any non-technical features (e.g. computer program, business method, aesthetic appearance, etc.) that do not contribute to the technical character of the invention may appear in the objective technical problem to be solved as a constraint that is to be met. This ensures that only the innovation in a technical field (as opposed to innovation in a non-technical field) is considered when assessing if an invention is obvious. For example, a claim to a new type of computer running an innovative business method contains technical features (the new computer) and non-technical features (a computer program for the innovative business method). In this case the objective technical problem to be solved might be formulated as the provision of a computer for running that innovative business method. Here, regardless of how innovative the business method might be in the field of commerce, it is not taken into account when assessing if the technical invention (i.e. the new computer) is obvious in view of the closest prior art.
6.3 Stage 3: Would it have been obvious?
The third and final stage of the PSA is to consider if the claimed invention would have been obvious: that is, would the skilled person, looking to solve the objective technical problem, have adapted or modified the closest prior art in a way that leads to the claimed invention? When looking to solve the objective technical problem the skilled person generally looks to his own and neighbouring technical fields for solutions. The solution may be part of the common general knowledge, it may be derivable from the closest prior art itself, or it may be found in another prior art disclosure (usually a second prior art document). Regardless of where the potential solution might lie, the Boards of Appeal have long recognised that, once an invention is known, it can often be shown how the skilled person could have made it by combining different teachings in the prior art (an “ex post facto” analysis). This kind of analysis must be avoided since it draws on knowledge of the invention. Therefore, to avoid hindsight creeping in at the final stage of the PSA the so-called “could-would” approach is routinely applied to consider if an invention is obvious.
6.3.1 Could-Would Approach
Rather than ask if the skilled person could have carried out the invention, the “could-would” approach requires that one ask if the skilled person would have carried out the invention in the expectation of solving the objective technical problem. In essence, the point is not whether the skilled person could have arrived at the invention by modifying the closest prior art, but rather whether, in expectation of the technical effect to be achieved (i.e. in the light of the objective technical problem to be solved), the skilled person would have done so using his common general knowledge or because of promptings in some other prior art disclosure.
One important caveat to this general “could-would” approach at the EPO is where the objective technical problem is to provide an alternative to that which is already known from the closest prior art. In this case the alternative invention can be viewed as one variation among a number of possible variations of the closest prior art. The Boards of Appeal have repeatedly stated that the simple act of arbitrarily selecting one variation among any number of equally obvious alternative variations is devoid of inventive character. It follows that the “could- would” approach (which requires that some motivation be shown for why the skilled person would consider a particular solution) is not particularly suited to situations where the objective technical problem is to provide an alternative. Instead, for these situations an invention is considered to be obvious if the feature that is missing from the closest prior art is already conventional for the technical field in question. Of course, even if the feature is shown to be conventional there must be no teaching away or incompatibility issues as otherwise the skilled person would not have modified or adapted the closest prior art to include it. The reader will appreciate that where the objective technical problem is to provide an alternative, the reduced burden to provide motivation generally makes it easier to establish that the invention is obvious.
6.3.2 Reasonable Expectation of Success
An important factor in determining if the skilled person would consider modifying the closest prior art in view of another prior art teaching is that he has a reasonable expectation that the modification will solve the objective technical problem. A reasonable expectation of success is something more than a “hope to succeed” as arguably all developments are made in the hope that they succeed. At the same time, the skilled person can be expected to tolerate some failure and so does not need to be certain of success. Whether there is a reasonable
expectation of success is very much dependent on the technical field in question and the case in hand. In comparatively unpredictable fields, such as chemistry or biotechnology, it is oftentimes more difficult to establish a reasonable expectation of success in the absence of a direct link between the feature in question and how it can help to solve the objective technical problem.
6.3.3 Bonus Effect
A surprising technical effect is generally indicative that an invention is not obvious and therefore can be considered as involving an inventive step. However, there are certain cases where the surprising effect is considered to be a bonus effect that arises naturally from that which is already obvious. For example, where it is considered obvious to modify the closest prior art to achieve a particular effect, the fact that the effect is achieved to a surprising degree is considered to be a bonus effect that does not make the invention any less obvious.
Furthermore, where it is obvious to modify the closest prior art to achieve a particular effect, and there is only one way to do this (the so-called “one-way street”), then any secondary effect arising from that modification is considered to be a bonus effect. Which effect is considered crucial and which is merely accidental (the “bonus effect”) requires a realistic approach to be taken that takes into account the relative technical and practical importance of those effects in the circumstances of a given case. For example, imagine that the application for the blue squash ball is primarily concerned with reducing visible markings on the white walls of a squash court, and that improved trackability is something that is discussed only briefly. If it could be shown that it is obvious to the skilled person that blue is the only colour other than green that reduces visible markings, then it could be argued that providing a blue squash ball is obvious and that the improved trackability of the blue ball is merely a bonus effect arising from that which is already obvious. However, if it could be shown that colours other than green and blue would also reduce visible markings (i.e. there is no one-way street but instead a choice of possible solutions to this particular problem) then the fact that the blue ball additionally leads to improved trackability is an effect that can be relied upon to argue that the invention is not obvious.
6.3.4 Other Considerations
Secondary indications such as a providing a solution to a long-felt need or enjoying commercial success can be taken into account when assessing if an invention is obvious. However, secondary indications of this kind are only of importance in cases of doubt, i.e. when objective evaluation of the prior art teachings has yet to provide a clear picture as to whether an invention is obvious.
7. Problem Inventions
It is the normal task of the skilled person to be constantly occupied with eliminating deficiencies, overcoming drawbacks and improving known devices and/or products. Therefore, the appreciation of conventional technical problems which form the basis of the normal activities of the skilled person, such as the removal of shortcomings, the optimisation of parameters or the saving of energy or time, do not involve an inventive step. However, in certain cases the discovery of an unrecognised problem may give rise to patentable subject-matter even if the claimed solution is retrospectively trivial and in itself obvious. In the squash ball example from above, one might argue that squash had been played for over one hundred years with the same black ball but nobody had appreciated that tracking a black ball against a white wall could be improved. Therefore, even if it could be shown that it was retrospectively obvious to improve trackability by making the ball blue, discovery of the unrecognised problem itself may provide basis for a patentable invention. That does not mean to say, however, that the absence of a hint in the prior art that there might still be a desire for further improvement means that an unrecognised problem has been discovered. The appreciation of a technical problem only contributes to inventive step in very exceptional circumstances.
8. Summary
Critical to the EPO’s evaluation of inventive step is the need to establish what contribution the invention makes in view of the closest prior art and to assess whether the skilled person is motivated by the state of the art to modify the closest prior art when seeking to provide that contribution. The EPO applies the PSA (i.e. “problem-solution-approach”) which formulates this contribution as the objective technical problem to be solved. Supporters of this approach argue that it allows for an objective assessment of the invention which only takes into account the actual technical contribution that is made with respect to the closest prior art.
Critics argue that the objective technical problem can be artificial (e.g. by choosing a single closest prior art) and is divorced from reality (e.g. by combining two pieces of prior art to reach the invention). Either way, there is no doubt that following the three stages of the PSA provides a reasonably efficient and predictable means by which to assess if an invention is obvious.
III. Prior Art
- Definition of the State of the Art
- Prior Rights under Art. 54(3) (EPC 2000 only)
- Non-Prejudicial Disclosure
1. Definition of the State of the Art
Under the EPC, the “state of the art”, or prior art, is defined in connection with the novelty requirement. The intention of the EPC is to prevent anything from being patented which is already a part of the state of the art. Thus, paragraph (1) of Art. 54 considers an invention “to be new if it does not form part of the state of the art”. Paragraph (2) then goes on to define the state of the art as comprising “everything made available to the public by means of a written or oral description, by use, or in any other way, before the date of filing of the European patent application”.
In defining the state of the art, the EPC applies an absolute concept, i.e. there are no restrictions whatsoever in regard to the place where the information concerned was made available to the public, or the language or manner in which this occurred. There is also no restriction in time (age), provided the information was made available before the filing date of the patent application concerned.
There are two, or rather three, exceptions to this absolute concept. Firstly, a European prior right, even though published only after the filing date of the patent application concerned, will be deemed to be part of the state of the art (Art. 54(3)). Secondly, there are two types of non-prejudicial disclosure which, even though publicly available, are not to be taken into consideration for the definition of the state of the art, provided that certain conditions are met (Art. 55).
1.1 Date of Disclosure
As regards the cut-off date for what constitutes prior art, Art. 54(2) refers to the “date of filing of the European patent application”. From the expression “date of filing”, it is derivable that a day is the minimum unit of time. Hence, it can be assumed that any disclosure of information on the same day as the filing date of the application does not form part of the state of the art (even if, e.g., the disclosure occurred in the morning and the filing only took place in the afternoon of the same day).
The “date of filing” as referred to in Art. 54(2) is the date on which the documents filed by the applicant contain (a) an indication that a European patent is sought,
(b) information identifying the applicant, and (c) a description or reference to a previously filed application (Art. 80 and R. 40(1)). When priority of an earlier application has been claimed in a valid manner, the date of priority will count as the date of filing (Art. 89).
The EPO will generally accept an indication in a document of the date of its publication as correct, unless sound reasons for contesting this are given (Guidelines B-VI 5.6). If the date of disclosure cannot be identified precisely, e.g., “ca. 1960”, such disclosure may still constitute prior art if it occurred far earlier than the relevant filing date (T 267/03).
If a document, such as a diploma thesis or a copy of a journal, is received by a library, the mere arrival in the library does not yet render the document part of the state of the art. Usually, at the time of arrival, the document is not yet catalogued or otherwise prepared for the public to gain knowledge of the existence of the document and its content. Thus, the exact date of availability to the public will depend on the library routine used (T 314/99 and T 1137/97).
1.2 Availability
Generally, only information which has been made available “to the public” can constitute prior art under Art. 54(2). At the same time, ”made available” does not require that one or more persons actually became aware of the information. Instead, it is sufficient if the public had the possibility of accessing and, thus, gaining knowledge of the information.
1.2.1 Public Information
Public availability of subject-matter requires that members of the public were able to gain knowledge thereof and that there was no bar of confidentiality restricting the use or dissemination of such knowledge. For instance, the registration of German utility models in the Utility Model Register will be published (announced) in the Patent Bulletin. However, the content of a utility model can be viewed by the public via inspection of the Register already from the date of registration. As a result, a German utility model will belong to the state of the art as of its date of registration, and not only as of the date of announcement of the registration.
Information is deemed to be available to the public if at least one member of the public is in a position to (i) gain access to the information and (ii) disseminate it to others.
On the one hand, the word “public” does not necessarily refer to the man in the street. On the other hand, the member of the public gaining access to the information in question does not have to be a person skilled in the art, provided that the member is capable of further distributing the information to skilled members of the public.
Requirement (ii) does not mean that the information must actually have been disclosed to further members of the public; it is sufficient if the at least one member of the public was in a position to do so, i.e. not being under an obligation to maintain secrecy.
In the case of a written disclosure, requirements (i) and (ii) will usually be satisfied as soon as the document concerned can be picked up and disseminated further. In the case of an oral disclosure, there is another requirement, namely that the at least one member of the public is a skilled person, or at least understands the information. Only if this condition is satisfied will it be possible to divulge the information to further members of the public, including skilled persons.
1.2.2 Confidential information
Information can only be publicly available in the sense of Art. 54(2) in the absence of an obligation of confidentiality or to maintain its secrecy.
Subject-matter is not deemed to have been made available to the public if there is an express or tacit agreement on secrecy, or if the circumstances of the case are such that such secrecy derives from a relationship of good faith or trust (Guidelines G-IV 7.2.2). Good faith and trust are factors which may occur in contractual or commercial relationships. For example, the content of a business meeting is not deemed to have been made available to the public if the parties concerned understood it to be secret and no breach of secrecy has been established. An understanding of this kind may be reached at a business meeting in the context of a joint technical development if the parties are assumed to have parallel interests and if there is a secrecy proviso agreed verbally and based on a stamp on a drawing (T 830/90).
However, even an existing non-disclosure agreement may not necessarily prevent certain subject-matter from becoming prior art, namely if the subject-matter has been further disseminated to the public in violation of the agreement (see item 3.1 below as regards the exception of Art. 55(1)(a)).
Despite the above, it should be noted that information does not become confidential for the sole reason that it is offered only with an obligation not to further disseminate it, as it is the case with many literature databases today. In fact, it is not necessary that the information be supplied free of charge, or that the recipient should be entitled to disseminate it, provided others can obtain the information for themselves from the original source (T 50/02).
1.3 Types of Disclosure
Art. 54(2) imposes no limitation on the means of disclosure, which is in line with the EPO’s absolute concept of the state of the art. Thus, the disclosure could be in writing, by oral presentation, by display of an object, or by (public) use or conducting of a method or process.
1.3.1 Written Disclosure
The usual form of prior art is generally a written document, and ordinary examples of such documents include patent documents and scientific or technical papers. However, any other form of written disclosure will be accepted, for example brochures, newspaper articles, or even handwritten notes taken, e.g., during a presentation.
While the existence of a written disclosure as such may not be in dispute, this may not apply to the time of its publication, or the question of its public availability (see 1.1 and 1.2 above).
As regards patent documents, they usually contain an indication as to their date of publication, so that the time of public availability will not be at issue. The EPO will, however, clearly distinguish between the publication of an application (A1, A2, etc.) and the publication of the granted patent (B1, B2, etc.), as the patent specification may include information not yet contained in the underlying application text.
The language of a document has no relevance on whether or not it constitutes prior art. In case of a patent document in a non-EPO language, the EPO may accept or cite a patent family member in an EPO language (English, French and German) for reference purposes, but the authoritative text and disclosure will always be the original document.
1.3.2 Oral Disclosure
A lecture or presentation, if made prior to the relevant filing date, can provide prior art, even though it may be difficult to ascertain the exact content of disclosure. Likewise, relevant state of the art may be generated by means of radio or television broadcasts.
Sometimes information is made available both orally (for example in a public presentation at a conference) and in writing (such as a subsequent scientific paper or conference proceedings). If only the oral presentation occurred before the relevant date, it cannot be assumed as a rule that the subsequent written document is identical in content to the oral presentation (T 1212/97). Written notes taken by an attendee of a conference will not automatically be accepted as prior art, as they are usually not made available to the public and may not be an accurate account of what was in fact conveyed to the public. Similarly, the lecturer’s script may not exactly reflect what was actually presented to the audience.
Finally, it may be disputed whether or not the oral disclosure was made unconditionally in public, i.e. such that arbitrary members of the public were able to take notice.
1.3.3 Public Prior Use
State of the art may also be generated by producing, offering, marketing or otherwise exploiting a product, or by offering or marketing a process or its application or by applying the process. In this regard, marketing may be affected by sale or exchange.
When an object is displayed on the occasion of a trade fair or similar event, it is generally accepted that only those features of the object are made available to the public which are visible from the outside. Internal features of the object which can be recognized only upon opening or even dismantling the object are deemed to be non-public, unless proof to the contrary is available.
For the use of an object or process to constitute part of the state of the art, the EPO will usually determine the following details (see Guidelines G-IV 7.2):
(i) the date on which the alleged use occurred, i.e. whether there was any instance of use before the relevant date;
(ii) what has been used, in order to determine the degree of similarity between the object used and the subject-matter of the European patent or patent application; and
(iii) all the circumstances relating to the use, in order to determine whether and to what extent it was made available to the public, as for example the place of use and the form of use.
These matters are oftentimes summarized as the When, What, How, Where and by Whom, and a public prior use will usually be acknowledged only if there is proof beyond reasonable doubt (T 2010/08).
1.3.4 Other Forms of Disclosure - Internet Disclosure
More recently, disclosure of information on the internet or in online databases has become a relevant source of prior art. Such information is considered to be publicly available as of the date on which the information was publicly posted. The EPO has published a specific Notice [1]setting out the EPO practice when citing documents retrieved from the internet. In view of the transient nature of online information, as compared to, e.g., a written document, it may be more difficult to establish, under the standard of balance of probabilities, (i) when a certain disclosure occurred and (ii) whether the content in question was actually made available to the public as of that date.
Generally, unless there are specific indications to the contrary, computer- generated timestamps will be considered by the EPO as reliable publication dates, and the frequently used “last modified” date will be accepted as the publication date. Where an explicit indication of the publication date is not available, or is unreliable, evidence for publication may be derived from an internet archive service such as the so-called “Wayback Machine” (www.archive.org).
If a web page is in principle available without any bar of confidentiality, neither restricting access to a limited circle of people (for instance via password protection) nor requiring payment for access will prevent said web page from constituting part of the state of the art (Guidelines G-IV 7.5.1).
Sometimes the internet publication of a scientific journal predates the printed paper publication, and the internet publication may deviate from the printed publication, for example lacking the final revision of the author. Thus, the EPO will usually treat the internet publication on the one hand and the printed publication on the other hand as separate documents having separate publication dates and possibly separate contents.
1.4 Scope of Disclosure
The content of a prior art document is to be interpreted with the eyes of the skilled person at the time the document was published. For instance, when the meaning of certain terminology changed over time, the terminology used in a prior art document must be given the meaning which it had at the time of publication.
1.4.1 Inherent Disclosure
If an object is provided unconditionally to a member of the public, the public is allowed an unlimited access to any material aspect of the object, including knowledge of any of its properties. That is, not only those features of the object are publicly available which may be ascertained from an external examination, but also those which require further analysis or testing.
For example, public sale of a piston made from a specific alloy will also make available the inherent, specific composition of the alloy in terms of types and amounts of constituent elements, or its specific gravity. In contrast, if the piston is merely displayed at a public exhibition, and the public can only view the piston, but has no possibility of analyzing the underlying alloy (being barred from taking the piston with it), then the composition of the alloy will not be part of the state of the art.
A document disclosing a (low molecular) chemical compound and its manufacture normallymakes available thiscompoundtothepublicinalldesired grades ofpurity, provided that such degree of purity could be accomplished with conventional purification methods in this field (T 990/96).
1.4.2 Enabling Disclosure
For a prior art disclosure to have been made available to the public, it is necessary that the information given to the skilled person is sufficient to enable him, at the relevant date, to carry out the technical teaching of the disclosure, taking into account the general knowledge at that time (the relevant date will be the date of publication or, in case of prior art under Art. 54(3), the date of filing). In other words, the disclosure belongs to the state of the art only if the skilled person can reproduce that subject-matter using common general knowledge.
For example (see Guidelines G-IV 2), a document may describe a chemical compound, either by name or structural formula, and indicate that the compound may be prepared by a process also described in that document. If the document does not indicate the details of the process, including starting materials and reaction conditions, and if the skilled person cannot get hold of the starting materials and suitable process conditions on the basis of his common general knowledge, e.g., relying on textbook knowledge, then the document would be regarded as providing an insufficient (non-enabling) disclosure with respect to said compound. Insofar, the chemical compound would not constitute part of the state of the art. If, however, the starting materials are commercially available, are well-known or described in reference text books, and if the skilled person can likewise identify suitable process conditions, using common general knowledge, the compound will be regarded as sufficiently disclosed. As a result, in this case, it would form part of the state of the art.
1.4.3 Erroneous Disclosure
If a prior art reference contains an error, such error will not affect its relevance as prior art provided that the skilled person, using common general knowledge, can
(i) readily recognize that there is an error and (ii) identify what the only possible correction should be. In such case, the document can be regarded as including the correction, and its disclosure should be interpreted accordingly, i.e. in a corrected manner (T 591/90).
In contrast, if the literal disclosure of a document is erroneous and does not reflect the intended technical information, such disclosure will not be included in the state of the art (T 77/87). In similar manner, published information may not be counted as forming part of the state of the art if the skilled reader will reject it as erroneous because it is evidently implausible (T 412/91).
2. Prior Rights under Art. 54(3) (EPC 2000 only)
Under the EPC, the state of the art also comprises the content of other European patent applications which were filed (or validly claim a priority date) earlier than the relevant date of filing of the patent application concerned, but which were only published on or after said date. These European patent applications constitute prior art only in the assessment of novelty, but not when considering inventive step (Art. 56). The aim of this provision is to prevent double patenting, accounting for the fact that a later applicant could become aware of the content of such a “prior right” only at the time of its publication, and not already at its date of filing.
2.1 Requirements
For a European patent application to constitute prior art under Art. 54(3), it must have a filing date which is before the relevant date of filing of the application concerned, and its publication date must be on or after that date. In addition, it is important that the prior right was still pending at the time of publication (it might be published despite its earlier withdrawal, namely when the withdrawal occurred at a time when the preparations for publication were already complete and, thus, publication could no longer be prevented) (J 5/81). Any changes in the state of the application after publication have no bearing on its effect as a prior right.
2.2 Scope
The “content” of a prior right is the whole disclosure of the application, including its description, drawings and claims, but excluding the abstract or the content of a priority document (both of which not being part of the application). The effect under Art. 54(3) covers all contracting states, independent of payment of a designation fee or withdrawal of designations. The effect also covers PCT applications designating EP, provided that the PCT applicant paid the required filing fee and, where applicable, supplied a translation into one of the official languages (English, French and German) to the EPO (Art. 153 and R. 159(1)(c)).
National rights of an earlier date in a Contracting State cannot constitute state of the art under Art. 54(3). However, they may constitute a ground for revocation under national patent law after grant (Art. 139(2)). For this reason, if a relevant prior national right exists, the applicant may be allowed to submit separate claims for the Contracting State concerned (see Guidelines H-III 4.5).
2.3 Toxic (Poisonous) Priority and Divisional Applications
Recent EPO practice has entertained the relatively new concept of a “toxi priority”. According to certain case law, if a European patent application claimed priority from an earlier application, and either (i) the earlier application is a European patent application and was published, or (ii) a divisional application was filed and published, the priority or divisional application was in some cases found to be capable of constituting novelty-destroying prior art under Art. 54(3). A parent application could also destroy the novelty of its own divisional application. This was the case if a generic claim term was broader in scope and, thus, not fully supported by the priority application. A specific embodiment contained in the priority application and published either as such or, e.g., in a divisional application might be cited as a novelty-destroying disclosure. A priority, divisional, co- divisional or parent application could therefore be “toxic” to other applications in the same family.
With decision G 1/15 of November 29, 2016 (published in full on February 1, 2017), the EPO Enlarged Board of Appeal has, however, effectively put an end to that practice, at least in all usual and foreseeable circumstances. That is because in G 1/15 the Enlarged Board has ruled that a generic claim that has been broadened relative to the priority application is entitled to priority partially, simply to the extent that subject matter falling within its scope was disclosed in the priority application. Even if the remainder of the broadened generic claim scope is not entitled to priority, the priority application, or e.g. a divisional or parent application to the extent its disclosure corresponds to the priority application and is thus entitled to priority, is therefore not citeable as prior art at all. For further discussion of this issue, see chapter on Priority of this book.
3. Non-Prejudicial Disclosure
The EPC does not provide for any grace period which would allow an inventor to disclose his invention prior to the filing of a patent application without said disclosure becoming state of the art. As explained in item 1.1 above, as a last resort, if an own disclosure occurred one day, and if this was noticed sufficiently early, then it might in principal be possible to prepare and file a patent application still on the same day, so that the earlier disclosure does not constitute detrimental prior art.
There are only two exceptions to the EPO’s absolute state of the art concept, thus excluding certain disclosures from the prior art, as explained below. However, they will not usually “save” an inventor who, of his own volition or by accident, disclosed his invention prior to filing a patent application. Hence, it is strictly advisable to make sure that any own disclosure of an invention be postponed until after the filing date.
3.1 Evident Abuse
According to alternative (a) of Art. 55(1), a disclosure will not be included in the state of the art if, subject to the 6-month time limit explained below, it was due to an evident abuse in relation to the applicant or his predecessor. This pertains in particular to any disclosure of the invention which occurred despite an express or tacit confidentiality agreement. An “evident abuse” will be established only if there was actual intent to cause harm or knowledge that harm would or could ensue from the unauthorized disclosure (T 173/83). Insofar, early publication of an invention as a result of an error may not necessarily satisfy the requirement of “evident abuse” (T 585/92).
3.2 International Exhibition
A second exemption of disclosure from the prior art is the display of the invention by the applicant (or his legal predecessor) at an officially recognized exhibition (Art. 55(1)(b)). “Recognized exhibitions” are published regularly in the EPO Official Journal. For validly making use of this exemption, a respective statement regarding the display of the invention is required at the time of filing of the application. In addition, the applicant has to file a supporting certificate within four months from the filing date (Art. 55(2) and R. 25).
3.3 Time Limit
Any disclosure to be excluded from the state of the art under Art. 55(1) must have taken place not earlier than six months preceding the filing of the application. For calculating the 6-month period, the relevant date is that of the actual filing of the European patent application, not the priority date (G 3/98 and G 2/99).
IV. Sufficiency of Disclosure
- Introduction
- Legal Basis
- The Person Skilled in the Art and Common General Knowledge
- Reproducibility
- Claim Scope
- Parameter Claims and Functional Features
- Pharmaceutical Inventions
- Deposit of Biological Material and Antibodies
- Evidence
- Conclusion
1. Introduction
As in any other patent law, the European Patent Convention requires the patentee to make the subject matter of the invention available to the public in return to the grant of the monopoly right.
2. Legal Basis
The basic legal provision for sufficiency of disclosure in the EPC is Article 83:
The European patent application must disclose the invention in a manner sufficiently clear and complete for it to be carried out by a person skilled in the art.
Since a European patent application may not be amended in such a way that it contains subject-matter which extends beyond the content of the application as filed (Art. 123(2) EPC), it is not permissible to add further information to the application at a later stage to rectify insufficiency issues. Hence, it is clear that the requirement of sufficiency of disclosure has to be met at the date of filing.
It should also be noted that Article 83 EPC not only refers to the description of the application, but to a European patent application as a whole. Therefore, European patent applications can be assessed for sufficiency of disclosure on the basis of their description, claims and their drawings, if any. In other words, it is not a requirement that any single part (e.g. claims) of the application should sufficiently disclose an invention.
Nevertheless, it is usually in the description that an invention is fully disclosed. The provisions relating to the content of the description are set out in Rule 42(1) EPC.
The description shall:
(a) specify the technical field to which the invention relates;
(b) indicate the background art which, as far as is known to the applicant, can be regarded as useful to understand the invention, draw up the European search report and examine the European patent application, and, preferably, cite the documents reflecting such art;
(c) disclose theinvention, as claimed, insuch termsthatthetechnicalproblem, even if not expressly stated as such, and its solution can be understood, and state any advantageous effects of the invention with reference to the background art;
(d) briefly describe the figures in the drawings, if any;
(e) describe in detail at least one way of carrying out the invention claimed using examples where appropriate and referring to the drawings, if any;
(f) indicate explicitly, when it is not obvious from the description or nature of the invention, the way in which the invention is industrially applicable.
Since the examiner of a patent application does not have the means to test and verify statements made by the applicant, an applicant may obtain a patent without facing any objections as to lack of sufficient disclosure. This may change in opposition proceedings, and in fact very often does, at least in cases relating to inventions in chemistry or biotechnology, as lack of sufficient disclosure is a ground for opposition (Art. 100(b) EPC).
3.The Person Skilled in the Art and Common General Knowledge
Article 83 refers to “the person skilled in the art”. This fictitious person determines whether the information as contained in the European patent application is sufficiently clear and complete to allow him or her to carry out the invention. The person skilled in the art is the same person that is used for assessing inventive step with the exception that when assessing sufficiency of disclosure, the person skilled in the art not only has knowledge of the prior art but also of the invention as disclosed (T 694/92).
The person skilled in the art is considered to resemble an ordinary practitioner aware of common general knowledge in the art at the relevant date, is capable of routine work and experimentation and is presumed to have access to everything in the state of the art, in particular handbooks and basic textbooks (cf. Guidelines for examination in the EPO, G-VII, 3).
It should be borne in mind, however, that the person skilled in the art is not inventive (T 39/93) and is a captive of established prejudices in his field (T 455/91). In an invention entailing multiple technical fields where multiple skilled people are required to perform an invention, the person skilled in the art may be considered to be a group of people skilled in their respective fields (T 141/87; T 99/89).
The skilled person’s common general knowledge will “automatically” be added to the described teaching. In other words, the assessment of sufficiency of disclosure must be based on both the written disclosure of the patent document as well as the common general knowledge available to the skilled worker.
The common general knowledge of the person skilled in the art has been defined by the Boards of Appeal as being normally represented by the content of encyclopedias, handbooks and dictionaries on the subject in question (T 766/91; T 206/83; and T 234/93). When a field of research is so new that technical knowledge is not yet available from textbooks, patent specifications and scientific publications have exceptionally been considered as belonging to the skilled person’s common general knowledge (T 51/97; T 772/89).
Moreover, the content of databases may belong to the common general knowledge, provided the following requirements are met (T 890/02): Firstly, the database should be known to the skilled person as an adequate source to obtain the required information. Secondly, the database is one from which the required information may be retrieved without undue burden, without the need of a search strategy. Thirdly, the database must provide the required information in a straightforward and unambiguous manner without doubts or need for further research work. These criteria were held applicable to all types of information sources, not only databases.
In decision T 890/02, the Board considered the information provided in the ENZYME and EMBL databases to belong to the common general knowledge, as the skilled person knows beforehand which type of information will be retrieved and can do so readily by inputting the name or EC number of the enzyme or nucleotide sequence. On the other hand, the Chemical Abstracts database was held to fail these requirements as a search in this database is a search over the complete prior art and anybody looking for information there is required to use sophisticated search strategies. Moreover, the kind of information retrieved cannot be anticipated before the search is made and more often than not, the skilled person would need to further pursue the original published articles.
In addition to his common general knowledge, the person skilled in the art may also obtain information required to reproduce the invention from another document which is cited in the original description (T 267/91; T 611/89).
4. Reproducibility
The European patent application must be sufficiently clear and complete to allow its reproduction.
According to Rule 42(1)(e) EPC, the description shall therefore “describe in detail at least one way of carrying out the invention claimed using examples where appropriate and referring to the drawings, if any”. Thus, examples are required where appropriate. However, if for example the patent specification gives precise instructions on what to do, examples may not be necessary (T 984/00).
The examples should confirm that the description of the patent application provides a coherent, convergent teaching enabling the skilled person to arrive at the goals of the claimed invention, and should thus make an extensive research programme superfluous. Examples should provide the person skilled in the art with all essential details necessary for the verification of their reported results (e.g. by repetition, if necessary), such as starting materials, process features and process conditions (T 1140/06).
If a specifically described example is not repeatable, this does not necessarily mean that the requirement of sufficiency of disclosure is not met (T 281/86). Rather, what is required is that the patent application describes the invention in sufficiently clear terms so that a person skilled in the art can reproduce it without “undue burden”. Even occasional failures and the necessity of trial-and-error experimentation does not necessarily render a patent application insufficient. A certain amount of trial and error is acceptable as long as a course of action is described that points in the direction of how to perform the invention even if negative results are initially obtained (T 322/93). However, trial and error becomes unacceptable if, on receiving a suboptimum result, no further direction is given as to how to perform the invention (T 727/95).
5. Claim Scope
The requirement of sufficiency of disclosure must be satisfied over the entire claim scope. The description of one way of performing the invention is thus sufficient only if it allows the invention to be performed in the whole range claimed. The question of whether one way is sufficient is a question of fact that must be answered on the basis of the available evidence, and on the balance of probabilities in each individual case (T 409/91).
When a claim covers a broad field or range, a patent application should not usually be regarded as satisfying the requirements of Article 83 EPC unless the description gives a number of examples or describes alternative embodiments or variations extending over the area protected by the claims (Guidelines F-III, 1). In such a case, the fact that the claim also covers non-working embodiments may not be harmful, and it is neither necessary nor appropriate to disclaim such non- working embodiments (G 1/03).
6. Parameter Claims and Functional Features
A product may be characterized by its parameters when the invention cannot be adequately defined in any other way, provided that those parameters can be clearly and reliably determined either by indications in the description or by objective procedures which are usual in the art (Guidelines F-IV, 4.11; T 94/82). In other words, when an invention is defined by a physical parameter, the requirement of sufficiency of disclosure is fulfilled only if the skilled person knows how said parameter can be measured with an acceptably accurate result, to an extent that any skilled person carrying it out will produce essentially the same result (T 541/97). If the gap between the explicit information about the measurement method provided in the patent and the necessary information to obtain acceptably accurate results cannot be filled by the skilled person’s common general knowledge or a cross-referenced document, the application fails for lack of sufficient disclosure (see e.g. T 805/93). Such a cross-referenced document is oftentimes an industry standard such as a JIS standard. However, such reference to industry standards should be used with caution as industry standards oftentimes do not specify all measurement conditions either.
Applicants also often use functional claim language with the aim of maximizing the protective scope and sometimes try to define the invention by a result to be achieved. Such claims are usually not allowed, in particular if they only amount to claiming the underlying technical problem (Guidelines F-IV, 4.10.). However, they may be allowed if the invention either can only be defined in such terms or cannot otherwise be defined more precisely without unduly restricting the scope of the claims. Moreover, the result must be one which can be directly and positively verified by tests or procedures adequately specified in the description or known to the skilled person and which do not require undue experimentation (T 68/85).
A functional definition of a compound by means of its binding to a receptor (“reach through claims“) lacks a sufficient disclosure if the identification of further compounds showing this function, besides those that are exemplified in the patent application, requires undue experimentation. If such further compounds can be prepared with routine methods (e.g. antibodies) or if there is a “selection rule“ for these compounds (T 1063/06), there may be no undue burden. A selection rule can possibly be a structure-activity relationship on the basis of which the skilled person could identify from the outset suitable compound classes, or the indication of a specific compound class (e.g. nucleic acids; T 216/96) in combination with the functional definition.
7. Pharmaceutical Inventions
In applications relating to pharmaceutical inventions where the therapeutic effect, such as the treatment of a specific disease, is in the claim, the patent application must make said effect plausible to fulfill the requirement of sufficiency of disclosure. If the effect is not in the claim (e.g. in case of pure compound claims), the lack of plausibility is a question of inventive step, rather than one of sufficiency of disclosure (G 1/03). The patent application should thus provide some information in the form of, for example, experimental tests, to the effect that the claimed compound, administered as stated in the claims, has a direct effect on a metabolic mechanism specifically involved in the disease (T 491/08). This mechanism must be either known from the prior art or demonstrated in the application per se.
What kind of experimental data is necessary thus depends on the type of disease and the knowledge of the metabolic mechanism involved in the disease. The experimental data may be obtained with cell cultures or immobilized biological markers/receptors in vitro or in silico or with animal models regarded as appropriate for reasonably inferring the possibility of a treatment of human beings. Data obtained with human patients is only required, if no established animal model exists (see e.g. T 1001/01; T 158/96; T 715/03).
In exceptional cases, the therapeutic effect may be plausible even without experimental data (see e.g. T 108/09, where the application however contained a detailed protocol of a planned clinical trial).
8. Deposit of Biological Material and Antibodies
It is oftentimes difficult, if not impossible, to adequately describe how to reproduce certain biological material. If an applicant files a patent for an invention using such biological material that is not available to the public, this can lead to a lack of sufficient disclosure. He must deposit the biological material in a recognised publicly accessible depositary institute. The relevant law for applications before the EPO is Rule 31(1) EPC.
According to this provision, the applicant should deposit cell lines and microorganisms under the Budapest Treaty at an international depositary authority such as the National Institute of Technology and Evaluation, Patent Microorganisms Depositary (NPMD) before the application’s effective date. If this is not (properly) done, the application may be insufficiently disclosed, which cannot be cured at a later point.
Thus, the sufficiency of disclosure of an antibody may be established by reference to a deposited hybridoma cell line. However, applicants should be aware that the reference to a single deposit may not be sufficient for a claim wherein the antibodies are defined by their function, e.g. their binding to a certain antigen; in such a case the requirement of sufficiency of disclosure may not be fulfilled over the entire claim scope (T 1466/05). If the function is the binding to a known antigen, the requirement of sufficient disclosure is however regularly met since the techniques for the production and selection of hybridomas are deemed to be common routine techniques.
Rule 31(1)(d) EPC regulates the conditions under which an invention involving the use of (deposited) biological material satisfies the requirements of sufficiency of disclosure when the depositor of such material does not correspond to the applicant of the patent application. If the depositor is a coapplicant of the EP application, then the provisions of Rule 31(1)(d) EPC do not apply. If the deposit was made by someone else, the requirements of this rule are fulfilled if the depositor has authorised one of the applicants (see Official Journal of the EPO 10/2010 par. 3.8).
For PCT applications published in an EPO official language (e.g., English), the indications of Rule 31(1)(d) EPC (name and address of the depositor, and the statement) must be furnished to the International Bureau (IB) within 16 months from the effective filing date. This time limit is deemed to have been met if the indication reaches the IB before the technical preparations for international publication have been completed (i.e. before entering the EP regional phase!) if they are not present in the application as originally filed. As a consequence of not complying with the requirements of R. 31(1)(d) EPC within this time limit, the application could be refused by the EPO examiner for insufficiency of disclosure. Further information and an example of depositor’s statement of authorisation and consent can be found in the Official Journal of the EPO 10/2010.
9. Evidence
The burden of proof to establish insufficiency of disclosure generally lies with an opponent in post-grant inter-partes proceedings or the examiner in ex-partes proceedings. An insufficiency objection generally presupposes that, in regard to enablement (T 19/90), there are serious doubts, substantiated by verifiable facts. When the granted patent does not provide any information of how a feature of the invention can be put into practice, only a weak presumption exists that the invention is sufficiently disclosed. In such case, the opponent can discharge his burden by plausibly arguing that common general knowledge would not enable the skilled person to put this feature into practice. The patent proprietor then has the burden of proof for providing a contrary assertion that common general knowledge would indeed enable the skilled person to carry out the invention (T 63/06).
Sufficiency of disclosure is a requirement that the EP patent application must comply with from the filing date. The insufficiency cannot be cured a posteriri, since this would add matter (contravening Art. 123(2) EPC). Accordingly, evidence published after the filing date cannot establish sufficiency of disclosure. For instance, if the description in a patent specification provides no more than a vague indication of a possible medical use for a chemical compound yet to be identified, more detailed evidence cannot be used later to remedy the fundamental insufficiency of disclosure of such subject-matter (T 609/02).
Evidence published after the filing date can however be taken into account to back-up the findings in the patent application, e.g. in relation to the use of an ingredient as a pharmaceutical (T 609/02).
10. Conclusion
Sufficiency of disclosure is a basic requirement for a European patent. While corresponding objections are not so common in examination proceedings, sufficiency of disclosure often plays a decisive role in opposition proceedings. This is particularly true in the fields of pharmacy and biotechnology, where most of the case law on this requirement has developed. This case law reveals differences with respect to approaches taken by other patent offices.
V. Patentable Subject-Matter I
- Introduction
- Inventions that Contravene “ordre public or morality” (Art. 5(a) EPC)
- Plant or Animal Varieties (Art. 53(b) EPC)
- The Exclusions of Medical Methods under Art. 53(C) EPC
1. Introduction
According to Art. 52 (1) EPC:
European patents shall be granted for any inventions, in all fields of technology, provided that they are new, involve an inventive step and are susceptible of industrial application.
In principle, thus, the EPC provides broad access to patentability for “inventions“ that provide a technical contribution to the art. The broad language of Art. 52(1) EPC is quite similar to Art. 27(1) of the TRIPS Agreement [2], which equally speaks of “any invention” in “all fields of technology”.
However, there are important limitations to patentability of certain kinds of subject matter. In fact, therefore, not “any” invention in “all” fields of technology is eligible to patent protection. The legislator’s motivation to exclude certain types of inventions from patent protection is as diverse as is the nature of the exclusions. It ranges from fundamental moral principles such as human dignity and the right to life, to more profane aspects like the avoidance of double protection for plant varieties under the EPC and plant variety protection acts. Other exclusions are motivated by socio-economic concerns, like the freedom of medical practitioners to treat patients, unimpeded by any patent rights.
In the EPC2000, the various exceptions to patentability are defined in Art. 53 EPC. The function of Art. 53 EPC is distinct from that of Art. 52 (2) and (3) EPC. Whilst Art. 52(2) and (3) defines subject matter that is not considered an invention in the first place [3], Art. 53 EPC excludes subject matter even though it is considered an invention, i.e. a technical contribution to the art.
Art. 53 EPC defines exclusions from patentability in three categories:
(a) inventions that contravene “ordre public” or morality
(b) plant or animal varieties or essentially biological processes for the production of plants or animals [4];
(c) methods for treatment of the human or animal body by surgery or therapy and diagnostic methods practised on the human or animal body [5].
These exceptions from patentability pursuant to Art. 53 EPC are similar, but not identical to the wording of Art. 27 (2) and (3) of the TRIPS agreement [6].
In practical terms, the consequences of these exceptions to patentability are very different. They range from the severe consequence of completely excluding certain subject matter from patent claims (e.g. business methods, surgical methods), to the much milder consequence of finding acceptable claim wording for (essentially) the same subject matter (e.g. methods of treatment).
The most frequently encountered exception from patentability concerns methods of treatment and diagnosis. As the EPC, however, allows patentability of products for use in methods of treatment/diagnosis (Art. 53(c) EPC, Art. 54(4) and (5) EPC), Applicants frequently have to adapt the claim language when prosecuting an application containing “method of treatment” claim language before the EPO. In certain constellations, finding appropriately amended language can be challenging.
In fact, the earliest decisions of Enlarged Board of Appeal (G1/83, G5/83, G6/83) were an interpretation of the exception of therapeutic methods. Ever since then, and including some recent decisions, the Enlarged Board had to further clarify the various exceptions under Art. 53 EPC.
The three sub-paragraphs of Art. 53 EPC and their interpretation by the Enlarged Board of Appeal will now be discussed in more detail.
2. Inventions that Contravene “ordre public or morality” (Art. 53(a) EPC)
In day-to-day practice, the exclusion of inventions contrary to “ordre public” or morality has, for a long time, been of little significance. The dogmatic discussion focused, forexample, onproductsordeviceswitha“dualuse”, i.e. havingpotentially “immoral”as well as “moral”applications. For example, a chemical agent may have the potential to kill human beings, but, on the other hand, may have a morally acceptable use, e.g. as intermediates in a production process. Similarly, certain devices may have (potentially) immoral applications, as well as morally acceptable ones. According to EPO practice, such products or devices having “dual use” areconsidered patentable. In other technical fields, e.g. weapons of mass destruction, it is not common to seek for patent protection, in the first place.
2.1 Art. 53(a) EPC in the Context of Biotechnological Inventions
More recently, however, with the advent of biotechnology, Art. 53(a) EPC and the respective rules in the Implementing Regulations have gained considerable attention. The field ofbiotechnology has been subject to– oftentimes controversial
– public debate, and, on the level of the European Union, has lead to the issuance of the so-called “Biotech directive” 98/44/EC of July 6, 1998. The aim of the directive was to harmonize the approach to biotechnological inventions across the member states of the European Union. Following this political aim, the EPO has adopted key aspects of the directive, such as the definition of “biotechnological invention”, into its Implementing Regulations (see definitions in Rule 26 EPC). The directive is to be used as a supplementary means to interpret the EPC (Rule 26(1) EPC).
Biotechnological inventions are such concerning “biological material” (Rule 26(2) EPC), which means material “containing genetic information and capable of reproducing itself or being reproduced in a biological system“ (Rule 26(3) EPC). Biotechnological inventions, in principle, are patentable under the EPC (Rule 27 EPC), even though there are important exceptions.
As concerns the exception from patentability under Art. 53(a) EPC, explicit guidance can be found in Rule 28 EPC, that lists the following biotechnological inventions as not patentable:
(a) processes for cloning human beings;
(b) processes for modifying the germ line genetic identity of human beings;
(c) uses of human embryos for industrial or commercial purposes.
The law-maker’s concern as regards human beings is further reflected in Rule 29 EPC, which excludes from patentability the “human body and its elements”. Not excluded, however, are elements isolated from the human body or produced by means of a technical process, even if these isolated elements are identical to that of a natural element (Rule 29(2) EPC). Thus, for example isolated (human) gene sequences may represent patentable subject matter under the EPC (Rule 29(2) EPC). The EPC imposes a limit, however, in so far as “the industrial application of a sequence or a partial sequence of a gene must be disclosed in the patent application” (Rule 29(3) EPC). This requirement follows suit from the restriction that the mere discovery of an element of the human body, such as a gene sequence, cannot constitute a patentable invention (Rule 29(1) EPC).
These exceptions in European practice do not, however, go as far as the current approach on natural products of the USPTO, following the controversial implementation of the decisions Alice v. CLS Bank, Mayo and Myriad into USPTO Examination Guidelines. In fact, Rule 27 EPC explicitly supports patentability of biological material isolated from its natural environment, even if it previously occurred in nature (Rule 27(a) EPC).
2.2 Patentability of Human Embryonic Stem (ES) Cells
The exception to patentability for moral reasons has been interpreted by the Enlarged Board of Appeal in its decision G2/06. Also subject of this interpretation was the guidance of Rule 28 EPC, in particular the prohibition to “uses of human embryos for industrial or commercial purposes” (Rule 28(c) EPC).
In its decision G2/06, the Enlarged Board of Appeal found that claims to human embryonic stem cells contravene Art. 53(a) EPC. The Enlarged Board reasoned that for the generation of human embryonic stem cells, it is necessary to destroy a human embryo, and that the destruction of a human embryo contravenes morality. More specifically, the Enlarged Board reasoned that the destruction of a human embryo to generate an ES cell line represents an industrial or commercial use, contrary to Rule 28(c) EPC. Some time after G2/06, the Court of Justice of the European Union (CJEU) was considering patentability of human embryonic stem cells in the so-called “Brüstle” decision (CJEU decision C-34/10). The CJEU took an even more restrictive approach than the EPO, in that it found that inventions are excluded that require:
the prior destruction of human embryos or their use as base material, whatever the stage at which that takes place and even if the description of the technical teaching claimed does not refer to the use of human embryos.
This reasoning excludes e.g. human embryonic stem cell lines, which were already established many years ago by destroying a human embryo.
Whereas the CJEU does not have formal jurisdiction over the EPO, the President of the EPO has decided to follow this decision, and has revised the EPO Guidelines for Examination (cf. G-II 5.3(iii); new part emphasised):
A claim directed to a product, which at the filing date of the application could be exclusively obtained by a method which necessarily involved the destruction of human embryos from which the said product is derived is excluded from patentability under Rule 28(c), even if said method is not part of the claim (see G 2/06). The point in time at which such destruction takes place is irrelevant (T 2221/10).
Thus, in the current practice of the EPO products exclusively prepared by the destruction of human embryos, independent of the point in time at which this takes place, are considered as “contrary to “ordre public” or morality” pursuant to Art. 53(a) EPC.
It should be noted that uses that are to the benefit of the embryo, e.g. a treatment of the embryo with the purpose of curing a disease, are not excluded from patentability under Art. 53(a) EPC.
From a practical point of view it is noteworthy that the EPO accepts that after January 2008 it was technically feasible to generate human embryonic stem cells without destruction of the embryo. This provides a technical counter-argument against an objection under Art. 53(a) EPC, for inventions having an effective date after this cut-off date. In other words, inventions on human embryonic stem cells having an effective date after January 2008 are potentially no longer excluded from patentability under Art. 53(a) EPC. Furthermore, it is worth noting that in the “Brüstle” case, the CJEU also ruled that any non-fertilised human ovum whose division and further development have been stimulated by parthenogenesis constitute a ‘human embryo’. However, soon after said decision, the CJEU took the exact opposite view in the “International stem cell” case (CJEU decision C-364/13), ruling that legal protection of biotechnological inventions must be interpreted as meaningthatanunfertilisedhumanovumwhosedivisionandfurtherdevelopment have been stimulated by parthenogenesis does not constitute a ‘human embryo’. This latter decision relied on the notion that parthenotes do not have the inherent capacity of developing into a human being. Following decision C-364/13, it appears that the EPO may even accept that before 2008 human embryotic stem cells were derivable from parthenotes without embryo destruction. One date which has already been admitted in EPO proceedings is June 5, 2003, based on the publication of WO 2003/046141, which discloses generation of human embryonic stem cells via parthenogenetically activated oocytes. The impact on Applicants of the decisions G2/06 and CJEU decision C-34/10 will therefore likely subside with time. Irrespective of patentability of the claims, the EPO will routinely request that the specification is adapted such that there is no reference to destruction of human embryos.
2.3 Suffering of Experimental Animals vs. Benefit to Mankind
Another landmark decision which concerned the exclusion of inventions “contrary to “ordre public” or morality” is the so-called “Oncomouse decision” (T19/90), dealing with the question whether a transgenic animal having an increased probability of developing cancer (or, in more general terms, a predisposition to a severe disease) is patentable under the EPC.
The relevant legal provision is the exclusion of “processes for modifying the genetic identity of animals which are likely to cause them suffering without any substantial medical benefit to man or animal, and also animals resulting from such processes”, pursuant to Rule 28 (d) EPC.
The competent Board of Appeal came to the conclusion that decisions on the patentability of transgenic animals will have to be made by “careful weighing up of the suffering of animals and possible risks to the environment on the one hand, and the invention’s usefulness to mankind on the other.” (cf. Reasons for the decision, item 5; emphasis added).
This “careful weighing up” is clearly case sensitive, and an assessment has to be made on a case-by-case basis.
3. Plant or Animal Varieties (Art. 53(b) EPC)
Art. 53(b) EPC excludes “plant or animal varieties or essentially biological processes for the production of plants or animals” from patentability, with the exception of microbiological processes.
3.1 The Exclusion of Plant and Animal Varieties
The EPC distinguishes between “plant or animal varieties” – which are non- patentable (Art. 53(b) EPC), and “plants and animals” in a wider sense, which are patentable (Rule 27(b) EPC), provided the “technical feasibility of the invention is not confined to a particular plant or animal variety”.
The scope of the exception to patentability is hence crucially dependent on the technical meaning of “variety”. As concerns plant varieties, the Implementing Regulations contain a definition in Rule 26(4) EPC:
“Plant variety” means any plant grouping within a single botanical taxon of the lowest known rank, which grouping, irrespective of whether the conditions for the grant of a plant variety right are fully met, can be:
(a) defined by the expression of the characteristics that results from a given genotype or combination of genotypes,
(b) distinguished from any other plant grouping by the expression of at least one of the said characteristics, and
(c) considered as a unit with regard to its suitability for being propagated unchanged.”
This definition is essentially the same as that used in the EU Regulation on Community Plant Variety Rights (EC 2100/94) [7]. This is consistent with the legislator’s intention to exclude parallel protection of plants a) as a plant variety, and b) in a patent. Thus, when the subject matter represents a plant variety, it can be protected as a plant variety right, when it is not a plant variety, it can be protected by a patent. This is sometimes referred to as a “seemless fit” between plant variety protection and patent protection.
Interestingly, whereas the law contains a clear definition of plant varieties, it does not with respect to animal varieties. Moreover, the exclusion of animal varieties requires a different justification, in so far as there is no codified protection for “animal varieties” that could lead to parallel protection.
In view of the economic significance of the plant breeding industry, the EPO had to decide on patentability of plant related claims on a number of occasions.
According to EPO practice, claims directed to a group of plants that may include, but is not identical to a plant variety, are allowable. This practice, set forth in G1/98, is consistent with the EU biotech directive [8]. The Guidelines for examination reflect this principle:
A claim wherein specific plant varieties are not individually claimed is not excluded from patentability under Art. 53(b) even though it may embrace plant varieties (see G1/98, and G-II, 5.4). (Guidelines, G-II 5.2(ii)).
In other words, a claim directed to e.g. “chrysanthemum variety White Snowball V” would not be patentable. A broader claim, directed to chrysanthemum plants having e.g. a certain genetic modification, on the other hand would be patentable under Art. 53(b) EPC, even if this claim covers “chrysanthemum variety White Snowball V”. Provided the correct claim wording is chosen, genetically modified plants are therefore patentable under Art. 53(b) EPC.
This approach has recently been confirmed in the parallel decisions G 2/12 and G 2/13, even for the case where the claimed plants can only be obtained by a “classical” crossing and selection approach, which in itself is excluded from patentability as an “essential biological process”.
As concerns animal varieties, much less case law is available. There is a consensus, however, that genetically modified animals do not represent an “animal variety”, and are not as such excluded from patentability under Art. 53(b) EPC.
3.2 The Exclusion of Breeding Processes
Breeding processes for plants or animals based on sexual crossing and selection are typically not patentable. There has been recent debate whether such processes can be patentable, if they comprise additional steps of technical nature. This discussion hinged on the wording of Rule 26(5) EPC, which defines “essentially biological processes” as such that “consist entirely of natural phenomena such as crossing or selection.” The discrepancy between the terms “essentially” and “entirely” in the wording of Rule 26(5) EPC lead to the parallel decisions G2/07 and G1/08 of the Enlarged Board of Appeal (the “broccoli” and “tomato” decisions).
The discussion is of relevance in the context of modern breeding techniques that are e.g. assisted by genetic markers used in crossing/selecting. The Enlarged Board held that the process does not become patentable under Art. 53(b) EPC, merely because such additional technical elements are claimed, as follows from the second headnote of G2/07:
2. Such a process does not escape the exclusion of Art. 53(b) EPC merely because it contains, as a further step or as part of any of the steps of crossing and selection, a step of a technical nature which serves to enable or assist the performance of the steps of sexually crossing the whole genomes of plants or of subsequently selecting plants.
Thus, processes “containing” a step of crossing/selecting are excluded from patentability, irrespective of additional technical elements. In justifying the exclusion, the Enlarged Board explained that the additional technical aspects may be elective to patent protection as such.
For example, a step of genetic modification, or the genetic marker suitable for assisting a crossing and selection process may be patentable subject matter in its own right, provided the claim does not include the sexual crossing and selection process [9]:
This is the case, for example, for genetic engineering techniques applied to plants which techniques differ profoundly from conventional breeding techniques as they work primarily through the purposeful insertion and/or modification of one or more genes in a plant (cf T 356/93 supra). However, in such cases the claims should not, explicitly or implicitly, include the sexual crossing and selection process.
However, in cases where the additional “steps of technical nature” are performed within – rather than before or after – the crossing and selection step, they may lead to patentability, as follows from the third headnote of G2/07:
3. If, however, such a process contains within the steps of sexually crossing and selecting an additional step of a technical nature, which step by itself introduces a trait into the genome or modifies a trait in the genome of the plant produced, so that the introduction or modification of that trait is not the result of the mixing of the genes of the plants chosen for sexual crossing, then the process is not excluded from patentability under Article 53(b) EPC.
The Enlarged Board of Appeal stresses that such additional steps of technical nature must be “performed within the steps of sexually crossing and selection… Otherwise the exclusion of sexual crossing and selection processes fro patentability under Article 53(b) EPC could be circumvented simply by adding steps which do not properly pertain to the crossing and selection process, being either upstream steps dealing with the preparation of the plant(s) to be crossed or downstream steps dealing with the further treatment of the plant resulting from such crossing and selection process” [10]. To ensure this limitation, the Enlarged Board of Appeal reasons that steps performed either before or after the process of crossing or selection should be ignored for determining the exclusion under Art. 53(b) EPC [11].
3.3 No Exclusion of Microbiological Processes
For completeness sake it should be emphasized that the exclusion of essentially biological processes does not extend to “a microbiological or other technical process, or a product obtained by means of such a process other than a plant or animal variety“ (Rule 27(c)EPC).
The term “microbiological process” means “any process involving or performed upon or resulting in microbiological material” (Rule 26(6) EPC). Hence, in the field of microbiology no exceptions to patentability in the sense of Art. 53 EPC are encountered.
4. The Exclusions of Medical Methods under Art. 53(C) EPC
Art. 53 (c) EPC contains three different exceptions to patentability:
- methods for treatment of the human or animal body by surgery,
- methods for treatment of the human or animal body by therapy, and
- diagnostic methods practised on the human or animal body.
All exceptions under Art. 53(c) EPC serve to free the work of medical practitioners from patent constraints. Under the EPC1974, the exceptions were based on a legal fiction of lack of industrial applicability. This legal fiction was given up in the revised EPC (EPC2000). It is acknowledged that the methods referred to in Art. 53(c) EPC are industrially applicable inventions. However, their exception from patentability is justifiable for reasons of public health.
Each of these exceptions has been subject to interpretation by the Enlarged Board of Appeal. The scope of the exceptions, and possible ways of overcoming the exceptions are quite different. The three exceptions will be discussed separately in the following.
4.1 Methods of Treatment of the Human or Animal Body by Surgery
For a long time, the EPO followed a restrictive approach when assessing this exception from patentability. In brief, this – out dated – approach of the EPO could be summarized as follows: If a method included any step of breaching the physical integrity of a person or animal, irrespective of how significant the breach was, the method was excluded from patentability. This also applied to relatively minor, routine interventions that did not require the presence of a medical doctor, such as e.g. venous puncture to draw a blood sample.
This strict, but simple, approach was significantly revised by the Enlarged Board of Appeal in its decision G1/07, which held that a narrower approach to the exclusion of treatments by surgery was required. According to this decision, the nature and severity of the surgical step is of significance.
Whether a method falls within the exclusion from patentability can be judged on the basis of the following criteria:
(a) The physical intervention is substantial.
(b) Professional medical expertise is required.
(c) Substantial health risk is involved.
Accordingly, the decision whether a particular surgical method is excluded from patentability has to be made on a case-by-case basis. This new approach relieves many types of subject matter from exclusion under Art. 53 EPC, which were previously excluded. Simple, routine interventions that are not associated with a significant health risk no longer justify an exclusion from patentability. On the other hand, it may not always be easy to predict whether a given claim is excluded from patentability, given the relative nature of the criteria set forth by the Enlarged Board of Appeal. It will be up to case law to provide further guidance whether a given physical intervention or health risk is “substantial”.
For instance, following G 1/07, the Board of Appeal ruled in T 663/02 that an intravenous injection of a magnetic resonance contrast agent, which can be delegated by a physician to a qualified paramedical professional, may be considered as representing a minor routine intervention which does not imply a substantial health risk when carried out with the required care and skill. The Board thereby found such a method step not to fall within the scope of Art. 53(c) EPC.
However, in contrast, in decision T 1075/06, issued shortly after T 663/02, the Board found that venipuncture of blood donors and the extraction of blood from a donor’s body represent substantial physical interventions on the body which entail a substantial health risk even when carried out with the required professional care end expertise. The Board thereby ruled that a method claim comprising steps encompassing such procedures is a method for treatment of the human body by surgery which is excluded from patentability under Article 53(c) EPC. Decision T 1075/06 also explicitly refers to T663/02 and states the assessment of the case would not have changed in light of said earlier decision.
Importantly, according to G 1/07, a single step of surgical nature in a multi-step process may suffice to trigger the exclusion (in this respect, previous practice was maintained), which was confirmed by T 1075/06. It is also irrelevant for this exclusion whether the surgical step is for therapeutic purposes. In this respect, the Enlarged Board of G 1/07 advocated a broad understanding of “treatment by surgery”, as referring to the nature of the treatment rather than its purpose.
This goes to show that there is currently no clear guidance from the EPO case law what type of surgical intervention will be acceptable, and even very minor interventions such as venous puncture to take a blood sample run the risk of triggering a respective objection.
When encountering an objection by the EPO, sometimes the claim can be reworded to avoid recital of a surgical method step. In case of a multi-step process, where only a single step is (allegedly) a surgical method step, it can be considered to define a particular patient group having received the surgical treatment, rather than the surgical method step as such. In the alternative, the omission of the surgical step has to be assessed. The concrete means of dealing with an objection against a surgical method step depend strongly on the nature of the invention, and on the relationship between the surgical step and the essential features defining the contribution to the art.
To give an example, a method may comprise a step of a) injecting a contrast agent into a patient, and b) obtaining an image from the patient. Such a claim could be reworded, such that it relates to taking an image from a patient, wherein the patient is characterized in that it has been administered with a contrast agent. In this wording, the claim does not comprise the injection step, but rather defines the appropriate patient group. This practice has been accepted by the EPO even under the stricter approach taken before G1/07. Such amendment may not be possible, however, if the sole technical contribution resides in the way of performing surgery, as such.
Devices used in surgical methods are patentable as surgical devices (i.e. products) per se. Their use in a method of surgery, however, would not be patentable. Moreover, surgical devices cannot be protected by a “second surgical use” claim, when the invention resides in a new surgical use of a known device. In this respect, practice on surgical methods is more restrictive as compared to pharmaceutical inventions (see below). The EPO reasons that surgical devices are not encompassed by the term “substance or composition” used in Art. 54 (5) EPC, and hence, devices are not eligible to purpose limited product claims. In principle, first or further surgical uses can be claimed for “substances or compositions”, analogously to first or further medical use claims (see below). However, such subject matter is only rarely encountered.
4.2 Exclusion of Methods of Therapy of the Human or Animal Body
In the field of pharmaceutical inventions, Art. 53 (c) EPC makes a strict distinction between “methods of therapy”, which are excluded from patentability, and “products, in particular substances or compositions”for use in such methods, which are patentable. The EPO considers use claims to be equivalent to method claims, such that use claims are equally excluded from patentability for pharmaceutical inventions. In a multi-step method, a single step that is of therapeutic nature is sufficient to trigger the exclusion under Art. 53(c) EPC.
The concept of “therapy” has to be understood broadly, and is not restricted to therapy for curative purposes. The Enlarged Board clarified in some of its earliest decisions thattheintentionof Art. 53(c) EPC was toprevent“medical and veterinary activities” from being restrained by patent rights (G 1/83, G 5/83 and G 6/83). This line of reasoning was maintained in G 1/07, confirming that the exclusion from patentability serves to protect the “medical and veterinary practitioners’ freedom to use the best available treatments to the benefit of their patients, uninhibited by any worry that some treatment might be covered by a patent”.
Only therapeutic methods are excluded from patentability, whereas for example cosmetic methods are not, i.e. the exclusion cannot be extended to treatments, which are not therapeutic in character. Where a treatment can have therapeutic or non-therapeutic effects, it may be necessary to clearly distinguish these in the claim, e.g. to explicitly direct the claim to a cosmetic method, to exclude therapy. Where the therapeutic and non-therapeutic use cannot be distinguished by the wording of the claim, a method claim will not be allowable.
Therapy generally relates to “the alleviation of the symptoms of pain and suffering”, as well as general or curative treatment of a disease. The concept of therapy also includes prophylactic treatment, aimed at maintaining health. In contrast, a method of contraception was not considered a method of therapy, as pregnancy is not an illness.
Whether prophylactic or curative, therapy must be “directed to the maintenance or restoration of health”.
4.3 Pharmaceutical Inventions
In the case of a novel substance, a pharmaceutical invention can be protected by a product claim. In contrast, where the substance or composition is known from the art, the claim needs to be restricted to the novel technical contribution – the use as a medicament. However, the claim cannot be drafted as a method or use claim, because of the exclusion under Art. 53(c) EPC.
In order to reflect the importance of research in the pharmaceutical field for society, the EPC provides an exception from the principle of absolute novelty in the pharmaceutical field. Specifically, Art. 54(4) and (5) EPC provide for purpose- limited product protection.
Specifically, a “substance or composition, comprised in the state of the art” can be protected “for use in a method of therapy”, provided that it has not been disclosed as a medicament in the art (Art. 54(4) EPC).
According to this provision, a composition or substance that has not previously been disclosed as a medicament can be patented in a so-called “first medical use” claim. According to the Guidelines for Examination, GII 4.2, first medical use claims can have the format: “Substance or composition X” followed by the indication of use, for instance “... for use as a medicament” or “... for use in therapy”.
This wording deviates from the practice required by the EPO immediately after the EPC2000 came into effect, where the indication of the use was to be worded “for use in a method of therapy…”
If a substance or composition has already been disclosed for use in therapy, Art. 54(5) EPC provides for so-called second- or further medical use claims, for any (novel) “specific use” in a method of therapy. The Guidelines for examination recommend a wording such as: “Substance or composition X” followed by the indication of the specific therapeutic use, for instance, “... for use in treating disease Y” (see G-VI 7.1).
Decision G 2/08 of the Enlarged Board of Appeal gives guidance on the correct interpretation of the term “specific”. The case underlying this decision was directed to the use of a known drug in a known treatment. The distinction over the art resided in a particular dosage regimen (administration once per day prior to sleep).
The Enlarged Board reasoned that a “specific” use in a method of therapy is not restricted to treating a novel disease, but extends to any “specific” aspect of treatment. This may be a particular way of treating the same illness, for example, a particular dosage regimen. It may also relate to a new patient group, within the more general teaching in the prior art, or, more generally, to a “new clinical situation”.
Thus, G2/08 opens a broad applicability of second/further medical indication claims under the novelty requirement. In practice, this oftentimes shifts the main challenge to patentability to the assessment of inventive step.
Additionally, decision G 2/08 holds that the so-called “Swiss-type claim” (“Use of compound X for the manufacture of a medicament for treating disease Y”) is no longer allowed for applications having an effective date after January 28, 2011.
Only the “purpose-limited product claims” as outlined above are allowable under the EPC2000.
4.4 Exclusion of Diagnostic Methods Practices on the Human or Animal Body
The final exclusion under Art. 53(c) EPC pertains to “diagnostic methods”, when “performed on the human or animal body”.
The Enlarged Board of Appeal gives guidance in G1/04 under which conditions a diagnostic method is “practised on the human or animal body”. Following this decision, a claim falls under the exclusion only when all of its steps are performed “on the human or animal body”. When a claim comprises at least one (technical) step performed in vitro, it does not fall under the exclusion. Thus, diagnostic method claims that encompass the in vitro determination of a marker or specific diagnostic value do not fall under the exclusion. For this reason alone, the practical scope of the exclusion is limited.
The assessment of (multi-step) diagnostic methods and surgical- or therapeutic methods is hence significantly different. A single surgical or therapeutic step suffices for a multi-step method to fall under the exclusion. In contrast, all of the steps of a diagnostic method must be performed on the human or animal body, to be excluded.
G1/04 in addition holds that, for a method to be excluded as a diagnostic method, it must also comprise the step of making the diagnosis in the stricter sense, or, in the words of the decision: “the diagnosis for curative purposes stricto sensu representing the deductive medical or veterinary decision phase as a purely intellectual exercise”. In contrast, methods that pertain to the steps preceding diagnosis, i.e. the data gathering or processing steps, but do not claim the diagnostic step as such, are not excluded. However, most diagnostic method claims are directed to the data gathering/processing aspect of the method, but do not necessarily include, as an essential feature of defining the contribution to the art, the “purely intellectual exercise” of the “medical or veterinary decision phase”, i.e. the diagnosis stricto sensu. For this additional reason, exclusions of diagnostic methods are quite rare in day-to-day practice.
Should an objection be raised, it can be assessed whether to omit the “medical or veterinary decision phase”, and/or add a limitation to in vitro for at least one particular method step.
In principle, purpose-limited product claims are allowable pursuant to Art. 54(4) and (5) EPC, for the first- or second diagnostic use of substances or compositions. However, such purpose limited product claims are only allowable if, in the first place, the method claim as such is excluded under Art. 53(c) EPC. For the reasons outlined above, this is quite rarely the case. Hence, in most cases it will not be possible to utilize purpose limited product claims in the diagnostic field. In these cases, however, method and use claims are permissible.
VI. Patentable Subject-Matter II
- Software Patents at the EPO: a Quick Guide
- Legal Provisions and Definitions
- What makes a subject matter technical?
- Examination of CIIs
- Eligibility
- The Modified Problem Solution Approach (PSA)
- Types of Claims
- Certain Aspects Relating to the Search Phase
- Some Recommendations
1. Software Patents at the EPO: a Quick Guide
Software inventions, or Computer Implemented Inventions (CIIs) as they are usually called at the EPO, are not excluded from patent protection if they are technical. However, inventive step of CII claims is acknowledged only on the basis of claim features providing a technical contribution.
Examination can be said to follow a two-stage approach. In the first stage, eligibility is affirmed if the claim defines at least a technical feature, without any reference to the state of the art and regardless of how trivial the technical feature is (in fact, a claim comprising a common computer would suffice to this effect). In the second stage, if novelty is established, inventive step is examined by focusing on those features which contribute to the technical character of the invention. Such features are determined on the basis of the technical effects achieved in the context of the invention. Features making no technical contribution, or any non- technical effect achieved by the invention, may instead appear in the formulation of the objective technical problem as a constraint that has to be met by the skilled person.
As a consequence of such an approach, overcoming the eligibility hurdle is relatively simple at the EPO. However, overcoming the inventive step hurdle may be relatively difficult in practice, since the claim may be stripped off of features not contributing to a technical effect.
While technical character is a central aspect to CIIs, there is no definition as to what is technical and what is not. Consolidated experience and in-depth knowledge of case law provide guidance in overcoming uncertainties and difficulties on a case- by-case basis.
2. Legal Provisions and Definitions
While software related invention is a generally understood expression, we will use the term Computer Implemented Invention (CII) as defined by the EPO:
An invention whose implementation involves the use of a computer, computer network or other programmable apparatus, the invention having one or more features which are realized wholly or partly by means of a computer program is termed a computer implemented invention. [12]
Evidently, the definition covers a wide range of software based innovations ranging from business methods to Graphical User Interfaces (GUI), from computer games to simulation software, from software control of industrial processes to software control of shopping systems, and in general to any solution implemented at least partly by means of a computer.
The general patentability requirements applicable to CIIs are given in Article 52(1) EPC setting out that patents are granted to inventions. No definition for invention is explicitly given, though. However, Article 52(2) EPC defines an exclusion list of what shall not be considered inventions, and thus not eligible to patent protection: (a) discoveries, scientific theories and mathematical methods; (b) aesthetic creations; (c) schemes, rules and methods for performing mental acts, playing games or doing business, and programs for computers; (d) presentations of information.
It is important to note that not only programs for computers, but all above listed items are relevant to CIIs, as in fact a mathematical method, a mental rule, a game, a presentation of information or a business scheme may all be implemented on a computer.
The exclusions from patentability shall however not be absolute, but (narrowly) applied only to “patent applications or patents that relate to said subject matter or activities as such”, Article 52 (3) EPC.
The Boards of Appeal have clarified certain implicit requirements of an invention, namely that it should solve a technical problem and be defined by technical features. The EPO often uses the term technicality to refer to technical character of an invention or to technical considerations underlying an invention. Based on this, the meaning of the exclusion list and of “as such” can be exemplified as that:
CIIs, even if directed to any item included in the exclusion list, can enjoy patent protection if they have technicality and if they are directed to a non- obvious technical solution.
3. What makes a subject matter technical?
As anticipated above, technicality plays a central role in establishing patent eligibility and inventive activity. The EPO has however refrained from giving a definition as to what “technical” means, on grounds that technological progress may soon render obsolete any such definition. Nevertheless, the following are examples elaborated from EPO case law, which could serve as guidance in assessing technicality of other cases:
- T208/84, VICOM: “a method of digitally filtering a two-dimensional data array having elements arranged in rows and columns” is not regarded as technical since it is directed to mathematical expressions (for instance, applying the convolution to a data array). However, “a method of digitally filtering images in the form of a two dimensional data array” may be regarded as technical, since it is an industrial application and as such belonging to the technical world. According to the Board, a “method for obtaining and/or reproducing an image of a physical object or even an image of a simulated object (as in CAS/CAM) may be used e.g. in investigating properties of the object or designing an industrial article”. Also, while mathematical methods operate on an abstract level, a technical process is one that is “carried out on a physical entity by some technical means implementing the method and provides as a result a certain change in that entity”, when noting that the entity can “be a material object but equally an image stored as an electric signal”.
- T110/90, IBM: a method “for transforming a source document cast in a first editable form which includes a plurality of input items therein, to a target document of a second form, comprising output items” would not be regarded as technical under EP practice, since this could also be exclusively executed mentally by a human being, possibly with the use of appropriate decision tables. Instead, an amended claim to a “method of transforming text which is represented in the form of digital data” comprising a step of “digitally processing the text” and further specifying that the input and output items refers to “input or output control items” may be considered technical. According to the Board, such a claim can “only be understood as meaning data in the form of digital electrical signals (bits and bytes)”, and thus not performable mentally; and that it “must be understood as acting on items of hardware such as a printer”, and not on abstract text items.
- T769/92, SOHEI discusses a claim directed to “a computer system for plural types of independent management including at least financial and inventory management”, further defining different types of files and processing operations performed on data comprised in said files in order to produce output data to be visualized. While the financial and inventory management of such a claim, when taken alone, could be regarded as being a business activity excluded from patentability, technicality is provided by the fact that the claim addresses technical considerations in that “the system allows data necessary for one type of processing … and data necessary for another type of processing to be performed independently … to be input using a single, common form … displayed to the user”.
Further to the above examples, the following can be regarded as rough indicators of the presence of technical character:- the problem solved by a feature or by a combination of features is technical;
- the claimed solution provides technical improvement(s) to a technical device or process;
- technical features and means are necessary for carrying out a method or process;
- technical considerations are required, especially when solving a technical problem by means of a technical feature.
4. Examination of CIIs
It can be said that examination is carried out in a two-stage approach: first eligibility is determined; then, if novelty is established, inventive step is assessed. Technicality plays a role in both stages, as it can also be derived from the following.
5. Eligibility
When deciding whether an invention is patent eligible or not, Examiners analyse the claim as a whole and without taking the prior art into account.
If the claim as a whole does not have technical character, then the claim is rejected as excluded from patent protection under Article 52(2) and (3) EPC. This can occur, for instance, if the claim defines a pure abstract mental activity or a business scheme without any explicit intervention of technical means.
Since the assessment is made independently of the prior art, the eligibility test is passed by including in the claim even the most trivial technical features like a computer or a pen. It is thus important for the application to provide support for the technical features necessary for the implementation of the invention.
From the above, it should be evident that the eligibility hurdle is relatively low at the EPO.
6.The Modified Problem Solution Approach (PSA)
The low eligibility hurdle shall however not be misleading: obtaining a patent for a CII may in fact still be difficult, since features not contributing to technicality can be decisive when assessing inventive step.
Case law has established that patents should be granted to inventions making a technical contribution over the prior art, or leading to technical improvements.
As such, inventive step is based only on those features that make a technical contribution.
In view of the above, and of the fact that CII claims may present a combination of technical and non-technical features, the EPO has modified the PSA to ensure, as far as possible, that patents are granted to inventions providing an inventive contribution to technology.
The modified objective technical problem (OTP) is schematically illustrated in the EPO Guidelines, which we have here slightly modified for illustrative purposes:
1) To start with, the features which contribute to the technical character of the invention are determined “on the basis of the technical effects achieved in the context of the invention”.
2) Based on the features identified as contributing to the technical character of the invention, the closest prior art is selected.
3) The distinguishing features over the closest prior art are determined, as well as the corresponding technical effects “in the context of the claim as a whole”. At this point, when having in mind the determined technical effects, a discrimination is made between those features which make a technical contribution and those which do not.
(a) If there are no differences (not even a non-technical difference), an objection of lack of novelty is raised.
(b) If the differences do not make any technical contribution, an objection under Art.56 is raised. The reasoning for the objection should be that the subject-matter of a claim cannot be inventive if there is no technical contribution to the prior art.
(c1) If the differences include features making a technical contribution, then the objective technical problem is formulated on the basis of the technical effect(s) achieved by these features.
(c2) In addition, if the differences include features making no technical contribution: these features may appear in the formulation of the objective technical problem as part of what is “given” to the skilled person, in particular as a constraint that has to be met. Similarly, any non-technical effect achieved by the invention may appear in the technical problem as a constraint to be met.
(c3) Once the technical problem is formulated, possibly taking into account the non-technical constraint(s), it is determined whether the claimed technical solution to the objective technical problem is obvious to the person skilled in the art. The presence or absence of inventive activity is thus established.
It can be said that including non-technical features, even if claimed, into the OTP is effectively applied in derogation to the general principle of avoiding hindsight, according to which the OTP should not comprise claim features or even pointers thereto. By way of the modified approach, however, the EPO ensures that even if a claim may easily pass the eligibility test, the same claim will lead to grant only if it provides a non-obvious technical solution.
It is also noteworthy noting that, while the modified PSA has long been applied by the EPO, it has now been modified in the most recent Guidelines. In particular, the previous version of the modified PSA allowed Examiners to start examination by identifying the “non-technical aspects” as claimed or as derivable from the description, and by formulating a requirement specification on the basis of the non-technical aspects. Thereafter, Examiners were asked to consider the (remaining) technical aspects, and assess whether the (remaining) features are inventive.
The new version of the modified PSA has deleted any reference to “non-technical aspects”, and apparently puts the initial examination focus on the features actually claimed, as well as their technical effect within the context of the invention. Only after the technical aim of the invention has been put into the spotlight, is it then possible to determine which are those features which do not contribute to technical character.
It can be said that the older version of the modified PSA allowed examination to start directly with depriving the claim from non-technical aspects. In contrast thereto, the new version of the modified PSA requires a higher initial attention to the claim features and their technical effect, before non-technical features can be stripped from the same claim.
The amendments to the Guidelines can theoretically mean that Examiners need to be more careful before dismissing certain features as non-technical. Practice will show whether these amendments will lead to any change in practice, and more importantly whether it can lead to higher legal certainty and predictability in this field.
7. Types of Claims
A CII invention can and should be claimed in different forms, for instance as method, apparatus, system, signal, computer program, medium for storing the program as long as the set of claims complies with the requirements of Rule 43.
It is however noted that, for a computer program claim not to be excluded from patentability, it is necessary to show that it is capable of bringing about, when running on or loaded into a computer, a further technical effect going beyond the “normal”physical interactions between the program (software) and the computer (hardware) on which it is run, as presently defined in the EPO Guidelines. In practice, if a method claim is considered allowable, then also a computer program claim referring to the method or defining features corresponding to those of the method would be allowable.
8. Certain Aspects Relating to the Search Phase
When dealing with CII inventions, and especially those having abstract or business related aspects, it is not unusual receiving a notification that no search could be carried out. This can occur when the claims are considered to be devoid of technical character. In other situations, the Examiner may regard the claim as defining only trivial technical features, like a computer; in this case, no prior art may be cited.
The applicant has however the possibility, when entering examination, to amend the non-searched claims in order to specify features lending technical character, and/or to argue in favor of technicality.
If the examining division is of the opinion that the initial deficiencies have been corrected by amendment or by convincing arguments provided by the applicant, it can still perform an additional search which may result in new prior art relevant for the claimed subject matter. Also, it is not infrequent that Examiners cite new documents also in late stages of the procedure.
9. Some Recommendations
In an ideal world, and when wanting to maximize the chances of success, a CII invention for the EPO should be drafted in an EP application differently and independently from an application directed to the same solution and intended for other jurisdictions. In a real world where a single text is to be drafted for later prosecution in multiple jurisdictions, the following is worth noting.
Since amendments shall be based on the application as originally filed, it is very important to ensure that the application documents as originally filed clearly disclose the technical character of the invention, e.g. in terms of technical features providing a technical effect.
Also, it is recommendable under EP practice to be silent on possible non-technical benefits reached by means of the invention. Similarly, it is generally better not mentioning possible non-technical motivations behind the invention, like for instance the wish to provide an incentive to purchase by means of a better user interface.
Finally, particular care needs to be taken for inventions that can be regarded as a computer implementation of an idea stemming from pure non-technical considerations or motivations.
PART B Prosecution of European Patent Applications
I. Filing of EP Applications
- Introduction
- Filing of Direct EP Applications
- Filing of Euro-PCT Applications
- Filing of Divisional Applications
1. Introduction
When filing at the EPO, there are two major categories of applications for applicants to consider: 1) direct EP applications, or 2) Euro-PCT applications. While several of the requirements are the same, there are also distinct differences which separate these applications and how they should be handled. A third general category, that applies to both direct EP applications and Euro-PCT applications, is the possibility of filing a EP divisional application based on either the direct EP application or the Euro-PCT application as the parent application. The EP divisional application is in turn issued a distinct EP application number and is considered a separate and individual application.
The path required for properly filing an application at the EPO can be complicated and difficult to follow, as issues concerning formal requirements, fees and terms must all be considered. The EPO has issued two guides to assist interested parties in maneuvering through the criteria for successful filing at the EPO. For direct EP applications, the EPO has prepared “How to get a European patent”; for Euro- PCT applications the EPO has prepared ““Euro-PCT Guide”: PCT Procedure at the EPO”, which are both available as free PDFs on the EPO homepage. While these guides, in general, outline the prosecution at the EPO, they are particularly helpful in navigating the requirements for filing at the EPO.
Without intending to address all issues that may arise with filing at the EPO, the most important filing requirements are considered below for direct EP applications, Euro-PCT applications, and follow-up divisional filings.
2. Filing of Direct EP Applications
A direct EP application is an application which has not been accorded a European filing date and is filed directly with the EPO as an application for examination at the EPO. In general, the requirements of a direct EP application are laid down in Art. 78 EPC. Art. 78(1) EPC requires the application to contain:
- a request for the grant of a European patent;
- a description of the invention;
- one or more claims;
- any drawings referred to in the description or the claims; and
- an abstract.
Further, the application must satisfy the requirements laid down in the Implementing Regulations.
- methods for treatment of the human or animal body by surgery
- methods for treatment of the human or animal body by therapy, and
- diagnostic methods practised on the human or animal body.
Art. 78(2) EPC requires the timely payment of the filing fee and the search fee.
While the maze of requirements laid down in the Implementing Regulations can appear to be endless, important is Rule 40(1) EPC (in combination with Art. 80 EPC), which defines the date of filing of a European patent application as the date on which the documents filed by the applicant contain:
- an indication that a European patent is sought;
- information identifying the applicant or allowing the applicant to be contacted; and
- a description or reference to a previously filed application.
An interesting and newly introduced option with the EPC2000 for acquiring a filing date is the possibility of merely referring to a previously filed application. As explained in Rule 40(2) EPC, the filing date and number of that application and the office with which it was filed must be stated. Such reference shall also indicate that it replaces the description and any drawings. While this option provides a new basis for applicants to file with the EPO, additional requirements including timely filing of certified copies and translations create a complicated mix of constellations that must be considered but which go beyond the scope of this summary.
Once the application has been assigned a filing date, Rule 57 EPC, entitled “Examination as to formal requirements”, applies. The general points of examination of Rule 57 EPC are as follows:
- the application is in an EPO language (e.g. where an application is filed in a non-EPO language a translation must be provided within 2 months of filing);
- a proper request for grant of a European patent is provided;
- the application contains one or more claims;
- the application contains an abstract;
- the filing fee and the search fee have been paid;
- a proper designation of the inventor is provided;
- where appropriate, one or more claims to priority are provided;
- where appropriate, proper representation is provided (e.g. an applicant who does not have residence or place of business in a contracting state can file the application itself but needs (duly appointed) representation for all subsequent acts);
- the application meets the physical requirements (e.g. text / format requirements); and
- where appropriate, a sequence listing is provided.
An interesting outcome of the revisions made in the EPC2000 is the possibility of filing and/or paying for one or more claims either on entry into the European regional phase or after filing the application.
For example, if the claims are filed subsequent to the filing of the application but prior to the formal examination pursuant to Rule 57 EPC, the EPO will not send out an invitation to file claims (i.e. Rule 58 EPC). On the other hand, if claims have not been provided by the time of formal examination, the EPO will issue a Rule 58 EPC invitation to file claims, which specifies a two month term. In the event that this term is not met, the application will be refused (i.e. Art. 78(1)(c) EPC with Art. 90(5) EPC). Here, it is important to realize that further processing is ruled out, and thus not available for failure to respond to the two month term set in Rule 58 EPC (i.e. Art. 121 EPC with Rule 136(4) EPC), albeit re-establishment is available.
Similarly, claim fees may also be paid at a later date after filing of the application. For example, when the claims are filed with the application, or shortly thereafter, the claim fees due for claims exceeding than 15 (i.e. the EPO only charges claim fees for the 16th and each subsequent claim; Rule 45(1) EPC) may be paid within one month of filing the claims (i.e. Rule 45(2) EPC). In the event that this term is not met, the EPO will issue a communication requesting payment of the claim fees due and will set a second one month term. In the event that this second term is not met, the claim(s) concerned shall be deemed to be abandoned. The applicant, however, still has the opportunity to request further processing within a two month term to allow for the claims to be reinstated and thus considered for prosecution. A similar scenario is possible in regard to a reference to an earlier application, as well as after a Rule 58 EPC invitation to file claims.
Another example of extending the time requirements of Rule 57 EPC concerns the language of the application. As explained in Art. 14(2) EPC, the application can be filed in any language; a translation of this application into one of the EPO official languages (i.e. English, German or French) must however be filed within a two month term. If this two month term is not met, the application shall be deemed to be withdrawn. Here too, the possibility of further processing is ruled out (i.e. not available), but re-establishment is available.
While there are several requirements tobe observed, the EPC provides considerable leeway for applicants to correct possible deficiencies. For example, for the request for grant of a patent, the use of an appropriate form is mandatory (Form 1001, Rule 41 EPC). A deficiency in this respect can be remedied within two months of invitation (Rule 58 EPC). Again, further processing is not available, but re- establishment is available. This also applies for deficiencies in case of compulsory representation. Deficiencies regarding the physical requirements of the application documents, e.g. paper, layout, tables, margins, numbering reference signs etc. (Rule 57(i) EPC) may also be remedied within two months of invitation (Rule 58, further processing excluded, re-establishment available). With respect to a sequence listing (Rule 57(j) EPC), if required or if it contains deficiencies, the applicant has two months to correct it and pay a late furnishing fee (Rule 30(3) EPC, here further processing is available). The filing and search fees (Art. 78(2) EPC) are due within one month of filing (Rule 38 EPC / Rule 45 EPC). The page fee is due within one month of filing the application / first set of claims / copy of previous application (Rule 38 EPC). Failure to observe those time limits triggers a loss of rights (Rule 112 EPC), however, further processing is available on request. The designation/extension fee is due within six months of the mention of the publication of the search report (i.e. time limit to request examination).
3. Filing of Euro-PCT Applications
A Euro-PCT application is an international application which has been accorded an international filing date and designates Europe as a designated region. A Euro- PCT application is equivalent to a regular direct EP application.
Prosecution of a Euro-PCT application starts only after the 31-month period, however, it may be accelerated on request both in the international phase (i.e. early entry on request) as well as in the European regional phase (i.e. PACE program).
Similar to a direct EP application and Rule 57 EPC, the EPC lays down the requirements that are to be fulfilled on entry into European regional phase in Art. 153 EPC, combined with Rule 159 EPC. As an aid to assist applicants in fulfilling these requirements, applicants are encouraged to use Form 1200 for entry into the European phase, however, this is not a requirement. Briefly, Rule 159 EPC requires:
- supply, where applicable, the translation of the international application;
- specify the application documents, as originally filed or as amended, on which the European grant procedure is to be based;
- pay the filing fee;
- pay the designation fee if necessary;
- pay the search fee, where a supplementary European search report has to be drawn up;
- file the request for examination if necessary;
- pay the renewal fee in respect of the third year if the fee has fallen due earlier; and
- file, where applicable, the certificate of exhibition
As outlined above for Rule 159 EPC, the applicant is required to specify the application documents, as originally filed or as amended, on which prosecution is to be based. While this provision allows applicants to file amendments of the application on entry of the European regional phase, including, for example, entire replacement pages or even a complete set of amended claims, the strict criteria followed by the EPO as to whether inadmissible subject matter has been added (i.e. Art. 123(2) EPC) of course still apply.
In the event that the applicant, however, is not in a position to provide such amendments, particularly in the form of newly drafted claims, the EPC provides an additional opportunity to file such in response to the Rule 161 EPC invitation, which is issued shortly after filing with a six month term. In the event that the claims are more than 15, additional claim fees are due and which are to be paid, at the latest, by the Rule 161 EPC term (i.e. Rule 162 EPC). Here, further processing is available, albeit two requests for further processing would be necessary if a new set of claims exceeding 15 claims forms the basis for the request for further processing.
Depending on which designated office the applicant has elected for the ISA in the international phase, amendments / comments may be required in response to the EPO’s Rule 161 EPC invitation. For example, in the event that the applicant has elected the EPO as the ISA in the international phase, the applicant would be required to provide amendments / comments to a negative written opinion provided in the international phase in response to the Rule 161 EPC invitation. On the other hand, if claim amendments are provided on entry into the European regional phase, no comments to the negative written opinion are necessary in response to the Rule 161 EPC invitation. By following this strategy, detailed comments in regard to the written opinion may be postponed for a later stage of prosecution.
Where an application is not filed in one of the official EPO languages, the translation of the international application required under Art. 153(4) EPC must be filed (Rule 159(1)(a) EPC). The language of the translation becomes the language of the proceedings before the EPO. Further processing and re-establishment are available (PCT overrules Rule 136(3) EPC).
As with the direct EP applications, the applicant is provided opportunities to remedy deficiencies, if necessary. For example, the filing fee provided for in Art.78(2) EPC, including a page fee for any page above 35 (not including the sequence listing), must be paid within 31 months (Rule 159(1)(c) EPC), otherwise the application is deemed withdrawn and no longer has any prior right effect. Here, both further processing and re-establishment are available. Further, the applicant must pay the designation fee if the period under Rule 39 EPC (i.e. within six months of the date on which the European Patent Bulletin mentions the publication of the European search report) has expired earlier (Rule 159(1)(d) EPC). Designation of one or more contracting states may be withdrawn at any time up to the grant of the patent. If the designation fee is not paid in due time, the application is deemed withdrawn, further processing is available (fee 50% of designation fee). The extension fees must also be paid within the same period as the designation fee. Where a supplementary European search report is to be drawn up, the search fee additionally needs to be paid (Rule 159(1)(e) EPC).
According to Rule 159(1)(f) EPC, the applicant is required to file the request for examination provided for in Art. 94 (written request and payment of examination fee). If the examination fee is not paid in due time, the application is deemed withdrawn, further processing is available and the further processing fee is 50% of the examination fee. Additionally, the applicant is required to pay the renewal fee (Rule 159(1)(g) EPC) in respect of the third year provided for in Art. 86(1) EPC, if the fee has fallen due earlier under Rule 51(1) EPC (i.e. the last day of the month containing the anniversary of the date of filing of the European patent application). After expiry of the relevant period, the renewal fee can still be paid with 50% surcharge within a 6 month period from the due date (no further processing is available, re-establishment is also possible).
Additionally, it may be required to provide data concerning the inventor (Rule 163(1) EPC), number / copy of priority application (Rule 163(2) EPC), and/ or the applicant’s address, nationality and state of residence / principle place of business (Rule 163(4) EPC), file a sequence listing (Rule 163(3) EPC), appoint a representative (Rule 163(5) EPC), limit the application to one invention after a non-unity finding, file a certificate of exhibition or provide any search results for a priority application (all of these may be carried out on invitation by EPO with respective terms).
4. Filing of Direct EP Applications
A direct European application, a Euro-PCT application, or a divisional application derived from either a direct European application or a Euro-PCT application may be divided by filing one or more divisional applications. The divisional application, however, must be filed while the parent application is still pending. An application is considered to be pending up to, but not including, the date that the European Patent Bulletin mentions the grant of the patent.
While it is not possible to validly file a divisional application when the parent application has been refused, withdrawn or deemed to be withdrawn, it is possible to file a new divisional application after refusal of an application in examination proceedings if the time limit for filing a notice of appeal has not expired (i.e. two months of notification of the decision to refuse the application; Enlarged Board of Appeal decision G1/09). In general, the procedure concerning the divisional application is independent from the procedure concerning the parent application, and the divisional application is treated as a new application, with the exception that the effective date remains the filing date or the priority date, as appropriate, of the parent application. Also, the right to file a divisional application in respect of an earlier application is only available to the registered applicants for the earlier applications jointly and not to one of them individually.
Similar to a direct EP application, claims may be filed after filing the divisional application, even if the parent application is no longer pending. In such a case, the EPO will issue a Rule 58 EPC communication, inviting the applicant to provide claims on which prosecution may be based with a two month term. In the event that this term is not met, there is no possibility of further processing and the application will be refused.
Also, a divisional application may be filed by reference to the parent application. On the other hand, routine to European patent practice is for the applicant to file the divisional application again, in its entirety, with the exception that the original claims of the parent are included as embodiments in the body of the specification and no longer as claims. This ensures that all of the subject matter of the original application is provided for in the divisional application, in particular for purposes of Art. 123(2) EPC (i.e. added matter), while at the same time allowing for a different set of claims to be filed with the divisional.
Such a new set of claims need not be limited to subject-matter in the claims of the parent application, however, they may not be extended beyond the content of the parent application as originally filed, i.e. such that the skilled person is presented with information not directly and unambiguously derivable from parent (Art. 76(1) EPC). On the other hand, double-patenting may be an issue if the divisional application claims the exact same subject-matter as granted in the parent application (i.e. G1/05 and G1/06).
The divisional application is to be filed in the language of the proceedings (Rule 36(2) EPC) of the parent or the language in which the parent was filed (if the parent is not in an official EPO language). In the latter case, a translation is to be provided within two months of filing (Rule 36(2) EPC) or on invitation to rectify failure to observe the time limit (Rule 58 EPC). This period of two months is excluded from further processing, but re-establishment is available.
A divisional application is deemed to have been filed on the date of filing of the parent application and enjoys any right of priority (Art. 76(1) EPC). The priority documents need not be filed again. Filing and search fee must be paid within one month of filing (Rule 36(3) EPC) and the designation fee must be paid within 6 month of the mention of the publication of the search report for the divisional (Rule 36(4) EPC). All contracting states are designated states, which, at the time of filing the divisional, are designated for the parent (Art. 76(2) EPC)
II. Claims
1. Introduction
The claims of European patents and patent applications are of the utmost importance. They are the basis for the assessment of patentability before the EPO in examination as well as in opposition and appeal proceedings, and also in national invalidation proceedings. Also, they determine the extent of protection conferred by a European patent or patent application when it comes to infringement litigation before national courts. Compared therewith, the description and drawings may be used only for interpreting the claims (Article 69(1) EPC and the Protocol on the Interpretation of Article 69 EPC).
Herein, we will set out the key requirements for claims under European practice.
2. Requirements of EPC
In view of the fundamental function of the claims, the proper drafting of claims is very important. Also, it is not surprising that requirements with respect to contents and form exist which are taken quite seriously by the EPO. A well drafted set of claims should:
- comply with the formal requirements of the EPC;
- provide a broad scope of protection;
- allow for enough freedom for possible later amendment and limitation; and
- where possible, even foresee and include future developments.
A core provision of the EPC relating to the claims is Article 84 EPC, which reads:
The claims shall define the matter for which protection is sought. They shall be clear and concise and be supported by the description.
Thus, when selecting the claim language to define an invention, Article 84 includes four requirements which must be taken into account, i.e. that the claims:
- define the matter for which protection is sought;
- are clear;
- are concise; and
- are supported by the description.
In the following sub-sections, we will discuss these requirements in more detail.
2.1 The Claims shall Define the Matter for Which Protection is Sought
This requirement set out in Article 84 EPC is supplemented by Rule 43(1) EPC the first sentence of which states that the claims “shall define the matter for which protection is sought in terms of the technical features of the invention”. This means that statements relating to non-technical features such as commercial advantages should be avoided. Several types of technical features may be used to define an invention:
- Structural features, such as the description of an arrangement of parts in a mechanical device, apparatuses, or chemical formulae;
- Compositions defined in terms of the nature and/or amounts of the ingredients, such as alloys or pharmaceutical composition;
- Functional features identifying specific functions or effects of individual elements, such as a “means for supply power” or a “foaming agent” (if the function relates to the claimed teaching in its entirety it’s more a purpose of use feature; see below);
- Process features defining a technical activity;
- Intended use features, defining the claimed teaching in terms of specific purposes of use for which it must be suitable, such as a “mold for molten steel”; and
- Parameter features, such as the definition of a substance by its melting point.
However, the applicant cannot entirely freely choose which kind of features to use to define the invention. In particular, the Guidelines for Examination in the EPO state that functional features can only be used if a skilled person would have no difficulty in providing some means of performing the claimed function without exercising inventive skill. Parameter features can only be used where the invention cannot be adequately defined in any other way. Products can only be defined using process features (so called product-by-process claims) where there is no other information available in the application to define the product satisfactorily using structural, composition, or parameter features.
2.2 The Claims shall be Clear
The meaning of a claim should be clear to the person skilled in the art from the wording of the claim alone. The case law of the EPO has developed numerous guidelines on the meaning of “clear” in Article 84 EPC. Therefore, it is worthwhile to bear in mind the following points when drafting and prosecuting claims for European patent applications.
Good practice:
- Include all features which are essential for the invention, i.e. all of the features which are necessary for achieving the technical effect of the invention. This is especially important if the description states that such features are essential.
- Include detailed definitions of the measurement methods allowing the unambiguous definition of any parameters used in the claims.
- Make sure that the features are defined in terms of proper units and, where needed, the basis for units used (such as “%” can be wt.-%, vol.%, mol.-%, … , and molecular weights can be based on a weight average, number average, …).
Bad practice:
- Using relative terms such as “thin”, “wide” or “strong”. If unavoidable, make sure that these terms are defined either in the claims themselves or in the application.
- Using features which define the result to be achieved by the claimed invention. For example, using a parameter which defines the high stability of chemical composition as a claim feature, when the technical effect achieved by the invention is improved stability.
- Using vague terms such as “about”or“approximately”in the claims. If such terms are used, it is preferable that they be defined in the description.
- Using trademarks. Objections are generally raised to the use of such terms in the claims. Therefore, if these terms are used in the claims or description, they should ideally be accompanied by a generic description.
- Referring to the drawings or description in the claims.
2.3 The Claims shall be Concise
This requirement relates both to the claims in their entirety, and to each individual claim.
There is a financial motivation for applicants to file not more than 15 claims in a European patent application, as significant claims fees are due for the 16th and subsequent claims (as of March 2017: € 235 per claim up the 50th claim, and € 585 for each further claim). Therefore, applicants tend to restrict the number of claims filed, with the result that it is rare for the EPO to object that the claims are not concise in their entirety for containing too many claims.
Objections to the conciseness of individual claims may be raised where wording is repeated due either to typographical errors or the incorrect use of dependent claims. While the use of alternatives in claims is permissible, an objection may also be raised to claims containing such a large number of alternatives that it is difficult to determine the scope of the claim.
2.4 The Claims shall be Supported by the Description
The EPO interprets this requirement of Article 84 EPC as being both a formal and substantive requirement.
In order to fulfil the formal requirement, there must be literal basis in the description for each of the claims. This can normally be achieved by including a section of the description in which the subject matter of each claim is reproduced.
The substantive requirement is based on the fundamental principle of European patent law that that the scope of protection should be consistent with the technical teaching of the patent. As a result, the claims should not be broader than is justified by the invention disclosed in the description and drawings of the application. Claims are typically generalizations of the invention disclosed in the description, and the EPO position is that the claim may cover all obvious modifications of, equivalents to, and uses of the invention described. In particular, it is permitted that the claims be broad enough to cover all embodiments which can reasonably be predicted by the person skilled in the art to exhibit the technical effect of the invention.
The onus is on the Examiner to provide well-founded reasons that the skilled person could not extend the teaching of the description to the whole of the claimed subject matter, using routine methods of experimentation or analysis. According to the Guidelines for Examination in the EPO, the Examiner should support such objections with published documents. It is only once such reasoned arguments have been set out that the burden of demonstrating that the claim is fully supported switches to the applicant. To illustrate this point, we refer to the following example.
Claim:
- A process of treating plant seeds, comprising the step of subjecting the seed to a temperature of 1–2°C for 1 day.
Description:
- A general section repeating the wording of the claim and explaining that the claimed seeds deliver plants with improved resistance to low temperatures.
- An example relates to one type of plant seeds only, showing that the plants resulting from the treated seeds have improved resistance to low temperatures.
Objection:
- The Examiner objects that the claim is not supported by the description, and provides evidence that certain types of seeds rapidly irreversibly decompose at temperatures below 5°C.
In this situation, unless the applicant can provide convincing evidence or arguments that the process is generally applicable, the claim must be restricted to the types of plant seeds which can reasonably be predicted to exhibit the technical effect of the invention. It would not be enough to assert that the process is applicable to all plant seedlings.
Although such an objection can be raised under Article 84 EPC, it can also be raised under Article 83 EPC on the basis that the disclosure is insufficient to enable the skilled person to carry out the invention over the whole of the claimed scope. This requirement is discussed in more detail elsewhere in the present book, see the chapter on the Disclosure Requirement. Although the choice of Article 84 EPC under which this objection is raised is unimportant in Examination Proceedings, it is critical in Opposition Proceedings as Article 84 EPC is not a valid ground of opposition.
A consideration of the extent to which the effect of an invention is achieved across the scope of the claims is also relevant to the assessment of inventive step, as discussed in the chapter on Inventive Step.
Special requirements for support by the description apply where the technical features of the claims are defined by their function. If only one way of achieving this function is exemplified in the application, the Examiner may object that the claim is not supported by the description unless it can be argued that the skilled person would appreciate that other means could be used for the same function.
For example, if a claim feature relates to a “means for supplying power”, and the description discloses an embodiment wherein the “means for supplying power” is a battery, no clarity objection would arise if the skilled person would understand that a solar panel or “means for supplying power” could be used in place of the battery. If however, no means beyond the concrete example given in the description are known for carrying out the claimed function, then a clarity objection may be raised.
2.5 Procedural Matters
Clarity is first assessed by the Search Division before drawing up the European search report. If the Examiner considers that the European patent application is so unclear that it is impossible to carry out a meaningful search, the applicant will be requested to file a statement indicating which subject-matter should be searched.
This procedure is discussed in greater detail in the chapter specifically addressing the aspect of the EPO search proceedings below.
Clarity is also assessed by the Examiner during prosecution. If the Examiner considers the claims to be unclear and this deficiency is not remedied or overcome by argument, the application will be refused.
Article 84 EPC is neither a ground of opposition, nor a ground for revocation in national courts. As a result, clarity is only discussed in opposition proceedings to the extent that an amendment of the granted claims introduces a lack of clarity (so decided by the Enlarged Board of Appeal of the EPO in the decision G 3/14).
3. Kinds of Claims
3.1 Categories
There are two basic kinds of claim: those to physical entities (products, apparatus, system), or those to activities (method, process, use). In addition to these groups, claims on pharmaceuticals can also be drafted in a “medical use” format, directed to a compound for use in a method of treating a certain disease. Such claims are interpreted by the EPO as purpose-limited product claims.
3.2 One Independent Claim per Category
According to Rule 43(2) EPC, a European patent application may contain more than one independent claim in the same category only if the subject-matter of the application involves one of the following:
(a) a plurality of interrelated products,
(b) different uses of a product or apparatus,
(c) alternative solutions to a particular problem, where it is inappropriate to cover these alternatives by a single claim.
The EPO interprets the term“interrelated”in Rule 43(2)(a) to mean different objects that complement each other or work together. Examples of such objects are: a plug and socket; a transmitter and receiver; an intermediate and final chemical product; and a gene, a gene construct, a host, a protein, and a medicament.
“Different uses of a product or apparatus” as defined in Rule 43(2)(b) include claims directed to further medical uses when a first medical use is known, and claims directed to the use of the same compound for multiple purposes, such as for fortifying hair and promoting hair growth.
Examples of“alternative solutions to a particular problem, where it is inappropriate to cover these alternatives by a single claim” according to Rule 43(2)(c) are groups of chemical compounds, or two or more processes for the manufacture of such compounds.
At the search stage, if the Examiner is of the opinion that the claims to be searched contain more than one independent claim per claim category, the applicant will be invited to indicate the claims on which the search is to be carried out. If the applicant fails to provide such an indication in due time, the EPO will carry out the search on the basis of the first claim in each category (Rule 62a(1) EPC). If the applicant does not agree with the Examiner that the claims contain more than one independent claim per claim category, the burden of proof is on the applicant to provide convincing arguments that one of the three exceptions set out in Rule 43(2) EPC applies. For details we refer to the section on EPO search proceedings.
Even if no objection is raised during the search, an objection may still be raised during Examination.
3.3 Dependent Claims
Rule 43(4) EPC defines a dependent claim as any claim which includes all the features of any other claim. Under European practice, dependent claims may refer back to one or more independent or dependent claims, or to both independent and dependent claims. In addition, dependent claims may also refer to independent claims in two categories as alternatives.
The introduction of such multiple dependencies is a most valuable tool under EPC practice. Different from other jurisdictions multiple dependencies are not considered as multiple claims, and thus there is no requirement for paying additional claim fees when multiple dependencies are introduced.
As multiple dependencies are not permitted in some jurisdictions outside of Europe, applicants form outside Europe sometimes submit claim sets in which dependent claims have only single dependencies. However, due to the strict approach to added matter adopted by the EPO (see the chapter on Amendments), this can later cause problems when amending the claims to combine the subject matter of two or more dependent claims.
From the perspective of European law, a simple way in which the risk of such added matter objections can be reduced (at the time of filing outside of Europe, such as in the case of an International (PCT) patent application) is to include the subject matter of the claims as a series of “embodiments” in the description, in which the embodiments corresponding to the dependent claims include back- references to multiple embodiments. Support for amendments combining the subject matter of two or more dependent claims can then be found in the corresponding embodiments.
3.4 Alternatives and Options
As noted above, claim fees are due in respect of the 16th and each subsequent claim. Therefore, there is a strong financial motivation to reduce the number of claims in European patent applications. One way in which this can be achieved is to combine the subject matter of dependent claims as alternatives or options in a single claim. Such claims are allowable before the EPO as long as they do not introduce a lack of clarity.
4. Claim Interpretation by EPO
The EPO is principally concerned with assessing the patentability of claims. To do so, the Boards of Appeal of the EPO have developed a number of guidelines for claim construction.
- Each claim should be read giving the words the meaning and scope which they normally have in the technical field of the invention, unless the description gives the words a special meaning. As a result, if the description gives a term a special meaning, it is generally desirable to include this definition within the claims themselves, in order to fulfill the clarity requirements of the EPC, and also to ensure that the special meanings are taken into account during the search and examination.
- The claim should also be read in an attempt to make technical sense out of it: such a reading may depart from the literal meaning of the claims.
- A claim to an “Apparatus/product B for carrying out the process C” is construed by the EPO as covering any apparatus suitable for carrying out the process. As a result, a claim to “A mold for molten steel” would lack novelty over any mold which was suitable for holding molten steel. Meanwhile, because a plastic mold has a melting point lower than that of molten steel, it would clearly not be suitable for carrying out the claimed process, and therefore would not be novelty destroying.
- On the other hand, the claim “Process D for doing activity E” is not interpreted as covering any process suitable for doing activity E. Instead, the technical effect is interpreted as a functional feature of the method. A method which was suitable for doing activity E but was not put to that use in the prior art would therefore not take away the novelty of this type of claim.
- Meanwhile, a claim “Process F of making product G for achieving technical effect H” is construed by the EPO to mean that the method or process has to be merely suitable for that use. Consequently, a prior disclosure of a method F for making the same product G without an indication of the particular purpose of achieving a technical effect H would anticipate this claim.
- A claim to “The use of product J for achieving effect K” is regarded by the EPO as being equivalent to “A process for achieving effect K using substance J”. Thus the claim is interpreted as relating to a process and not to a product J recognizable (e.g. by labelling or additives) as intended for use for achieving effect K.
- In contrast, a claim directed to “the use of a process L for achieving the technical effect M” is interpreted as being equivalent to a claim directed simply to the process L.
- For applications having filing or priority dates before January 29, 2011, claims in the Swiss-type format “The use of a substance or composition for the manufacture of a medicament for a specified therapeutic application” are interpreted by the EPO as being novel if the substance, the method of manufacture, or therapeutic application was not disclosed in the prior art. This interpretation is only applied to substances or compositions for medical uses.
- For applications granted on or after December 13, 2007, claims in the first medical use format “Substance for use as a medicament” or the second medical use format “Substance for use in the treatment of disease” will be interpreted as purpose-limited product claims. As a result, first medical use claims are interpreted as novel if the substance has not previously been used as a medicament, while second medical use claims are interpreted as novel if the substance has not previously been used for that specific medical indication.
- Product-by-process claims are interpreted by the EPO as being novel only if the product itself is novel. Therefore, it is normally necessary to provide evidence or convincing arguments that the process defined in the claim leads to a product with distinct properties.
- Product-by-process claims in the format “Product M obtained by the process N” and “A product M obtainable by the process N” will be interpreted by the EPO as having equivalent scope. As a result, both claim formats would lack novelty over a prior art product which has the same properties as the product made by the process in the claim, even if that prior art product is made by a different process. As the scope of both claim formats is interpreted differently in some EPC contracting states, it is desirable to use the broader “obtainable” language in European patent applications.
- Optional features do not limit the scope of the claim. However, as discussed above, the introduction of such features into claims may be useful when reducing the number of claims.
5. Summary
The present section outlines some general and fundamental aspects of how claims should be drafted, what formal requirements have to be observed, and what types of claims and features for defining the teaching of an invention are available. Due to the central relevance of the claims in patents and patent applications there are many other aspects of patent practice which are in the one or the other way connected to/relative for the claims. These aspects, as far as not addressed here, are discussed in greater detail elsewhere in the present book, and questions not answered here might find an answer in these more specific chapters.
III. Priority
- Introduction
- Basic Requirements for Claiming Priority under the EPC
- Procedural Formalities
- Partial Priority - an Antidote to “Toxic Divisionals”
1. Introduction
This chapter provides an overview of the main aspects of claiming priority which are of practical importance for patent applicants in Europe. Well-settled, essential requirements are covered, as well as “partial priority” in the case of generic claim terms and the closely related issue of“toxic”self-collision of European applications within a single patent family (e.g.: “toxic divisional applications”), which has only recently been resolved by EPO Enlarged Board of Appeal decision G 1/15. The chapter also covers the question of the requirements for the valid transfer of priority rights, which is at present less well settled and may therefore in certain circumstances expose applicants to risks.
2. Basic Requirements for Claiming Priority under the EPC
The main principles of priority under the EPC are set out by Art. 87 (1) EPC which reads:
Any person who has duly filed, in or for (a) any State party to the Paris Convention for the Protection of Industrial Property or (b) any Member of the World Trade Organization, an application for a patent, a utility model or a utility certificate, or his successor in title, shall enjoy, for the purpose of filing a European patent application in respect of the same invention, a right of priority during a period of twelve months from the date of filing of the first application.
The terms shown in boldface above highlight six basic requirements for successfully claiming priority, namely that priority under the EPC can only be claimed 1) from an application that was duly filed in an eligible territory; 2) from an eligible type of application; 3) if the application claiming priority was filed within twelve months of the earliest claimed priority date; 4) by the same applicant who filed the priority application or by his successor in title; 5) in respect of the same invention; and 6) from the first application by the applicant (or by his predecessor in title) for the subject matter in question.
2.1 Basic Formal Requirements
Priority under the EPC may be claimed from applications filed “in or for” any of the currently 176 contracting states of the Paris Convention or the currently 161 member states of the World Trade Organization. Unlike Art. 4A(1) of the Paris Convention, which only provides for priority claims from applications filed in other countries than the country of the first application (external priority), the EPC also recognises internal priority, i.e. from previous European applications. Priority may also be claimed from PCT applications.
Priority may be claimed from no other types of intellectual property rights than the applications for patents or utility models/certificates as expressly recited in Art. 87(1) EPC. However, for these types of filing, the establishment of the valid filing date is sufficient to give rise to a right to claim priority. The subsequent fate of the priority filing is irrelevant. Priority may also validly be claimed, for example, from filings from which a patent has already been granted at the time the application claiming priority is filed.
If the application claiming priority is filed later than the 12-month maximum priority interval of Art. 87(1), the EPC provides that the application may be filed and re-establishment of rights requested within the following two months (Art. 122 and Rule 136 EPC).
However, such requests are successful only in exceptional circumstances, i.e. when the applicant satisfies the onerous requirement that the 12-month time limit was missed in spite of all due care required by the circumstances having been taken.
2.2 Applicant Identity and the Transfer of Priority Rights
Under Art. 87(1) EPC, if the applicant of the priority application is not the same as the applicant of a subsequent application claiming the priority, a claim to priority will be invalid if a valid succession in title to the right to claim priority cannot be established.
If one or more applicants are added at the time of filing the subsequent application, the joint filing and priority claim implies the consent of all parties that the priority right is to be transferred to the larger group of co-applicants. No further proof of transfer is then required. In all other cases of “applicant mismatch”, a valid transfer must be demonstrated. The EPC is, however, silent on the formal requirements for a valid transfer of a priority right, and the national requirements across Europe (and indeed the rest of the world) vary. This leads to legal uncertainty and non- uniform case law and practice both within the EPO and under the national practices across Europe.
Generally, it may be taken to be settled practice under the EPC that right to claim priority must be validly transferred before the subsequent application claiming priorityhas beenfiled. Deficiencies inthetransfercannotberemediedretroactively. This view has been confirmed, e.g., by recent English High Court decisions. In decision T 62/05, an EPO Board of Appeal applied very strict formal requirements to the transfer of priority rights, requiring that, just as for the assignment of a European patent application as such (Art. 72 EPC), the assignment of priority rights must be in writing and signed by both parties. As long as this approach is not expressly abandoned by the EPO, the best-practice recommendation can only be that the strict, formal requirements prescribed by T 62/05 should be adhered to for the assignment of any priority right which may in future become relevant under the EPC.
However, the approach that currently appears to be preferred by many EPO tribunals is that the transfer of a priority right – which under European practice is generally and in principle considered to be separate and independent from the transfer of a patent application as such –needs to be shown to be valid under the “relevant” national provisions. Such an approach is consistent with the absence of express provisions in the EPC and goes back to earlier EPO Appeal Board decision J 19/87. It will generally depend on (1) the determination of the country of the “relevant” national law, and (2) the provision of legal expert opinion evidence in order to establish how that national law applies to the case in question. Determination of the correct law to apply might be highly relevant, as the local law on the minimum requirements for an effective transfer of property differ from country to country, and within the US, perhaps even from state to state.
It currently does not yet appear to be finally settled under EPO practice by which principle the applicable national law is to be chosen in international constellations, e.g., if the parties to the transfer agreement are based in different countries and the priority application has been filed in yet another country, and the assignment perhaps under the application of the national law of yet a further country. In principle, the relevant law under this approach may be (1) the law of the country/ territory in which the priority application was filed, or (2) the law with which the contract governing the transfer of the priority right has the closest connection (which may vary depending on the particular circumstances of each case).
Generally, the third possible approach applies the law of the territory in which the later application claiming priority is filed. T 62/05 applied a form of this third approach, and this is also the approach taken under the national law of many countries. It leads to the problem that any agreement document governing the transfer of priority right must comply with the formal requirements for the transfer of priority in any territory in which protection might eventually be desired, even before any subsequent application claiming priority has been filed, i.e. in practice often long before any final decision is typically made on the territorial coverage that is required for a given invention (see e.g., Bremi, EPI Information 1/10:17-24 for discussion).
One recent and highly relevant decision on this point is T 517/14, which takes the position that the applicable law is the national law applicable to the transfer of the right of priority, i.e. the law governing the assignment contract.
Thus, in practice, applicants worldwide who eventually wish to obtain patent protection in EPC territory should, when transferring priority rights, adhere to the strict standard of T 62/05 and moreover to any requirements under national lawin individual countries in which protection may be desired or which are associated with either the priority application or the transfer agreement.
2.3 Same Invention
The EPO Enlarged Board of Appeal in decision G 2/98 interpreted the “same invention” requirement as requiring the same strict, formalistic “subject matter” or “disclosure” test which the EPO also applies to the questions of added subject matter in the case of claim amendments or divisional applications, and also when assessing novelty. Priority was thus only to be acknowledged for subject matter of a claim which the skilled person can derive directly and unambiguously, using common general knowledge, from the priority application as a whole.
More flexible standards that had sometimes been applied prior to decision G 2/98 were thus rejected. In a later decision (G 2/10), the Enlarged Board of Appeal has confirmed the uniform applicability of this “uniform concept of disclosure” (also referred to interchangeably as a “disclosure test” or a “subject matter test”) in the various above-mentioned areas of the law under the EPC.
2.4 First Application
This sixth basic requirement mentioned above is derivable, in particular, from Art. 87(1) in conjunction with Art. 87(4) EPC, which reads:
A subsequent application in respect of the same subject-matter as a previous first application and filed in or for the same State shall be considered as the first application for the purposes of determining priority, provided that, at the date of filing the subsequent application, the previous application has been withdrawn, abandoned or refused, without being open to public inspection and without leaving any rights outstanding, and has not served as a basis for claiming a right of priority. The previous application may not thereafter serve as a basis for claiming a right of priority.
Thus, a subsequent application containing subject matter that was previously filed in a “previous first application” can nevertheless be considered as the eligible “first application” for the purpose of validly claiming priority, if a number of further requirements are met, which essentially amount to the timely elimination of the “previous first application” including all of its legal effects, just as though it had never existed.
The Enlarged Board in G 2/98 also expressly equated the meaning of the expression “the same invention” in Art. 87(1) EPC with the concept of “the same subject-matter” to which Art. 87(4) EPC refers in the context of determining which filing is the relevant “first application” for the purposes of assessing entitlement to priority. The “first application” for particular subject matter is therefore also determined according to the above-mentioned disclosure test.
3. Procedural Formalities
According to Rule 52 EPC, the declaration of priority may in principle still be made within and corrected 16 months from the earliest priority date claimed. However, any such correction must be submitted within four months of the date of filing of the European application claiming priority, and a declaration of priority may not be made or corrected after a request for early publication of the application claiming priority has been filed.
By the same 16-month deadline, European patent applicants must file a copy of any priority application, certified by the authority with which the priority application was filed. The EPO waives that requirement in certain cases, i.e., currently, if the priority application is a Japanese, Korean or Chinese patent or utility model application; a US application; or an EP application; a PCT application filed with the EPO as receiving office.
Where the priority application is not in English, French or German and during any proceedings at the EPO the EPO considers that the validity of the priority claim may be relevant to patentability, the EPO requires a translation of that application into one of the official languages to be filed. Alternatively, a declaration may be submitted that the European patent application is a complete translation of the previous application. In the case of noncompliance with such an invitation, the right to priority in question is lost.
4. Partial Priority – an Antidote to “Toxic Divisionals”
4.1 Legal Basis and Enlarged Board Decision G 1/15
The claiming of multiple priorities and the concept of “partial priority” in the context of a single patent claim are provided for by Art. 88(2) EPC, second sentence, which reads:
Where appropriate, multiple priorities may be claimed for any one claim.
Although this provision only refers to “multiple priorities”, it has long been well recognized that it also applies to “partial priority” in a narrow meaning (Schricker GRUR Int 1967, 3), i.e., also if only a single priority is claimed. The priority date may then apply to one part of the subject matter of a claim, and the filing date to the remainder of the claim.
Regarding European patent applications claiming subject matter that was added after the date of at least one priority application, Art. 88(3) EPC also provides:
If one or more priorities are claimed in respect of a European patent application, the right of priority shall cover only those elements of the European patent application which are included in the application or applications whose priority is claimed.
This further confirms that a claim of a European patent application may cover subject matter going beyond what was disclosed in a priority application, and may then only be partially entitled to priority, namely only for subject matter which was disclosed in the priority application. A claim may thus in principle be split into “partial priority domains” of subject matter having different effective dates, depending on whether the subject matter in question is entitled to a particular priority claim.
Under EPO practice such “splitting” of the claim scope has not generally posed particular problems when the different domains have been expressly individualized as alternatives by the wording of the claim and these alternatives also correspond exactly to “elements” that either are or are not disclosed in a priority application. However, until the recent decision G 1/15 of the EPO Enlarged Board of Appeal (dated November 29, 2016 but published in full only on February 1, 2017) it was not clear under EPO practice whether such subject matter“splitting”was allowable when the possible “alternative subject matters” forming partial priority domains were not made explicit in the claim, but were identifiable within the scope of a generic claim term only conceptually, by reference to a narrower disclosure found in the priority application and merely encompassed within the scope of a broader generic claim term employed in the later filing that claims priority. Typical examples are the broadening of a chemical formula or of a numerical range, but the issue applies to any generic broadening of claim terms relative to the terminology of the priority application.
In G 1/15 of the EPO Enlarged Board of Appeal has now clarified that the concept of partial priority applies in a straightforward and relatively applicant/patentee- friendly manner to any claim term which has been broadened in a generic manner in comparison to the priority document. This decision also effectively renders obsolete the topic of “toxic divisionals” and similar cases of self-collision arising from “toxic priority”, which has troubled applicants and patentees in Europe for the larger part of the last decade. The approach to partial priority that has been confirmed in G 1/15 provides an “antidote” to such self-collision within the same patent family, at least in all usual and generally foreseeable circumstances.
Enlarged Board decision G 1/15 became necessary because after Enlarged Board decision G 2/98 the case law of the EPO Boards of Appeal on partial priority diverged into two irreconcialable approaches. According to one line of case law, which until G 1/15 also represented the approach generally applied by first-instance examining and opposition divisions, partial priority was not acknowledged for subject matter that had basis in the priority application but was not individualised as such in a generically broadened claim (e.g., T 1127/00, T 184/06, T 1443/05, T 1877/08 and T 476/09). This restrictive approach required different subject matter domains corresponding to different effective dates to be expressly identified as distinct alternatives by the wording of the claim, using the word “or”.
This approach was based on a restrictive interpretation of a statement found in previous decision G 2/98 of the Enlarged Board of Appeal, namely: “The use of a generic term or formula in a claim for which multiple priorities are claimed in accordance with Article 88(2) EPC, second sentence, is perfectly acceptable under Articles 87(1) and 88(3) EPC, provided that it gives rise to the claiming of a limited number of clearly defined alternative subject-matters” (point 6.7 of the reasons).
However, a different line of case law, represented by cases T 1222/11 and T 571/10 argued that the above restrictive approach failed to recognize that the Enlarged Board in G 2/98 took a teleological and historical approach to the interpretation of Art. 88(2) EPC, second sentence, and held that the legislative intent underlying this provision was expressed by a particular “Memorandum” drawn up by FICPI for the Munich Diplomatic Conference prior to the adoption of the EPC, on the issue of multiple and partial priorities in the case of a single claim. T 1222/11 and T 571/10 therefore followed the approach to this issue that is explained in the Memorandum, and interpreted the “proviso” of G 2/98, point 6.7 of the reasons (underlined above) entirely differently.
In G 1/15 the Enlarged Board of Appeal has now confirmed that this more lenient case law is correct, essentially adopting the reasoning of T 1222/11 and T 571/10. According to this “Memorandum approach”, when the terms in a claim have been broadened compared to the corresponding disclosure in the priority document, a partial priority entitlement simply applies to the extent that the claim scope corresponds to the disclosure of the priority document. The broadened, generic claim scope in question is thus “conceptually” divided into subject matter domains having different effective dates purely on the basis of the narrower disclosure in the priority document(s). This purely “conceptual” subdivision of a generic claim term into partial priority domains is independent not only of the wording of the claim as such but also of the content of the later application as a whole. Within generic claim terms that are not as such entitled to priority across their entire scope, the basis for any narrower subject matter that is entitled to priority is found in the priority document.
Under the former, restrictive approach which G 1/15 has rejected, when a generic term such as a chemical formula or numerical range was broadened with respect to the priority application, priority was lost entirely. Any intervening disclosure corresponding to the content of the priority application during the priority interval thus destroyed the novelty of the claim. In contrast, under the lenient “Memorandum approach” now confirmed in G 1/15, the priority entitlement is automatically maintained to the extent that subject matter is disclosed for the first time in the priority application, which will generally protect the claim from any intervening disclosure corresponding to or falling within the scope of the content of the priority application.
Therefore, Enlarged Board decision G 1/15 effectively removes the risks that have in recent years been posed to patent applicants and patentees in Europe by the concept of “toxic divisional applications”, “toxic published EP priority applications” and the like. In principle under the EPC, and particularly under the restrictive, now- obsolete case law discussed above, the above-mentioned intervening disclosure could be, e.g., content of a divisional, co-divisional or a parent application, to the extent that such an application from the same family was entitled to priority. It could also be priority application itself, if that was European application and allowed to be published. Any such related application could, in principle, be considered to be an entirely independent European patent application, such that any of its content that is entitled to the priority date may, once published, be considered to be earlier-filed/later-published prior art under Art. 54(3) EPC and thus relevant for novelty, if the later claim in question is for some reason not entitled to priority.
Under the restrictive approach that existed prior to G 1/15, priority applications (if European and published), and in any case any priority-entitled content of published divisional, co-divisional and parent applications could become “toxic” and destroy the novelty of claims which have been generically broadened beyond the priority application (cf., e.g., EPO Board of Appeal decisions T 1443/05, T 1496/11 and the discussion in T 557/13, the referring decision in G 1/15). However, following G 1/15, if a claim is generically broadened beyond the priority application, the priority-entitled disclosure of such related applications is not generally expected to become prior art at all, due to the automatic protection that is afforded by the lenient “Memorandum approach” to partial priority.
The referral to the Enlarged Board in G 1/15 also included the question, essentially, of whether, if not the concept of partial priority, any other aspect of the EPC prevented the citation of a parent or divisional application of a European patent application as prior art relevant for novelty under Article54 (3) EPC. The Enlarged Board held that, in light of its ruling on how partial priority was to be applied in practice so as to prevent such self-collision, it was not necessary to decide or comment on this question separately.
Considering at least that it is common practice (1) to improve inventions in the course of the priority interval and broaden the scope of the protection that is sought in a later application claiming priority and (2) to file divisional applications, the conformation of the “Memorandum approach” by the EPO Enlarged Board of Appeal in G 1/15 comes as a relief to patent applicants and patentees in Europe. It is now once again generally safe to file divisional applications in Europe and if desired to prosecute European priority filings further, to publication and grant if desired, at least in all usual and foreseeable circumstances. This conclusion is expected to apply not only at the EPO but in principle also to national practices in EPC contracting states such as Germany and the UK, which do take into account the practice of the EPO in such matters, particularly if case law of the Enlarged Board of Appeal exists.
4.2 The “flip-side” of the G 1/15 Approach
While the confirmation of the Memorandum approach is generally a welcome development for applicants, it also comes with a “flip-side”. This was made evident in T 1222/11, a decision which has been expressly confirmed as correct by the Enlarged Board in G 1/15. This “flip-side” of the Memorandum approach means that it is now it more important to plan patent portfolios such that the definitive European or international application for a given invention is filed within the twelve-month period after the earliest patent filing of the narrowest embodiment of the invention.
This is a consequence of the principle that partial priority / subject-matter domains may be identified “conceptually” within a generic claim scope by comparison with narrower disclosures in the priority application, in conjunction with the further basic principle mentioned above in section 1.4 that priority under the EPC can only be claimed from the first application by the applicant (or by his predecessor in title) for the subject matter in question.
The case underlying decision T 1222/11 revealed in practice how these two principles can work in conjuction to the detriment of patent applicants. To be consistent on all aspects of priority, the Board in that case applied the same approach of “conceptually” identifying subject matter domains within the scope of a generic claim or disclosure when assessing, by comparison with yet earlier applications, whether the priority application was in fact the first application for all “conceptually” identifiable priority domains its disclosure encompassed. A yet earlier-filed application by the same applicant that was published in the priority interval and disclosed a narrow embodiment encompassed by the definition used in both the priority application and the claim in question therefore ultimately destroyed the novelty of the claim. Priority could not be claimed in respect of the narrow embodiment because at least in the “partial subject matter domain” of that narrow embodiment, the priority application was not the “first application”.
IV. European Search
- Introduction
- The Extended European Search Report
- Supplementary European Searches
- Response to the Extended European Search Report
1. Introduction
The unit within the EPO that is responsible for carrying out the search and drawing up the search report for an application is the Search Division. The task of the Search Division is primarily to carry out searches and to draw up the European Search Reports in relation to European patent applications. The Search Division also carries the search and draws up the search report for Euro-PCT applications for which the EPO acts as designated or elected Office and which has been accorded an international date of filing, the so called supplementary European searches. In this chapter we will summarize the procedural matters concerning the European Search Report and the supplementary European Search Report.
2. The Extended European Search Report
2.1 The European Search Report
According to Art. 92 the EPO shall draw up and publish a European search report in respect of the European patent application on the basis of the claims, with due regard to the description and any drawings. Hence, the search is not restricted by the literal wording of the claims but the examiner is considering the content of the claims in light of the description and drawings. Further, where technical features which are not present in the claims are indicated in the description as essential for the solution of the stated problem, these features are included in the search. Insofar as possible the search also covers subject-matter to which the claims might reasonably be expected to be directed after they have been amended.
Before receiving the European search report, where the European application does not derive from an earlier international application (the so called direct European patent application) the applicant may not amend the description, claims or drawings of the European patent application. Accordingly, the search is directed to the claims as originally filed or the claims as filed under Rule 57(c) or 58.
The EPC does not provide for a time limit for issuing the European search report. However, the applicant may request an accelerated search under the program for accelerated prosecution of the European patent applications (PACE). In this case the EPO makes an effort to issue the search report as soon as possible. For European patent applications claiming no priority (first filings) the EPO carries out an accelerated search within six months of the date of filing without the need for a separate request. In such cases the applicant must indicate his intention not to file a declaration of priority.
Before starting with the search the examiner analyzes the claims of the European patent application in view of the requirements for technical character of the claimed subject-matter (Art. 52(2), (3)), non-exclusion from patentability under Art. 53, susceptibility of industrial application (Art. 57), conciseness and clarity of the claims (Art. 84, Rule 43(2)) as well as unity of the invention (Art. 82, Rule 44). Based on such analysis the examiner may exclude certain subject-matter from the search.
In the following we will discuss the procedural matters in case of non-compliance with these requirements.
1) If the patent application contains a plurality of independent claims in the same category and the examiner considers that the exceptional situations of Rule 43(2)(a)-(c) are not applicable (i.e. the examiner believes that the claims lack conciseness), the examiner will issue a communication under Rule 62a(1). This communication is an invitation for the applicant to indicate, within two months, the claims complying with Rule 43(2), on the basis of which the search is to be carried out.
In response to this communication the applicant may react in four different ways:
- If the applicant does not wish to dispute the examiner’s opinion, the applicant may indicate the independent claims that fulfill the requirements of Rule 43(2) and that he wishes to have searched.
- If the applicant finds the objection not justified, the applicant may contest the examiner’s opinion. The burden of proof to show that the claims indeed fulfill the requirements of Rule 43(2) lies with the applicant. Hence, in this situation the applicant must provide reasons why the requirements of Rule 43(2) are fulfilled. If the examiner is not convinced by the applicant’s argumentation, the examiner will search on the basis of the first independent claim per category. As an alternative, the applicant may as a main request, request that the claims as filed be completely searched by providing reasons why the requirements of Rule 43(2) are fulfilled, and as an auxiliary request, in the case the examiner is not convinced by the applicant’s arguments, indicate the subject-matter to be searched.
- The applicant may find the objection only partly not justified and wishes that part of the plurality of independent claims in the same category be searched. Similar to the above situation, the burden of proof lies with the applicant to show that that part of the plurality of independent claims satisfies the requirements of Rule 43(2). If the examiner finds the indicated claims not to comply with Rule 43(2), the examiner will search the independent claim with the lowest number indicated by the applicant.
- The applicant may also decide not to reply to the invitation. In this case the examiner carries the search on the basis of the first independent claim in each category.
The final responsibility whether the communication under Rule 62a(1) was justified lies with the Examining Division. During substantive examination, if the Examining Division finds the communication under Rule 62a(1) justified, it shall invite the applicant to restrict the claims to the searched ones (Rule 62a(2)). If in response the applicant contests the restricted search and the Examining Division is convinced by the applicant’s arguments, an additional search will be carried out at the examination stage.
2) In some cases the examiner may consider that the application does not comply with the requirements for unity of the invention (Art. 82). In such cases the examiner will initially only draw up a partial search report for the invention first mentioned in the claims (Rule 64(1) first sentence). The examiner will send the partial search report to the applicant together with an invitation under Rule 64(1) to pay a further search fee for each further invention to be searched. The partial search report will indicate how many different inventions the examiner considers to be present in the application, and which of the claims belong to each of the identified inventions. As from 1 April 2017, the EPO will start providing applicants with a provisional opinion on the patentability of the invention (or unitary group of inventions) first mentioned in the claims. This will be sent together with the invitation to pay further/additional search fees and the partial search results (see Rules 64(1) and 164(1)(a) EPC, and Article 17(3)(a) PCT).
The opinion that the application does not comply with the requirements for unity cannot be contested in the search stage. Hence, in reply to the invitation under Rule 64(1) the applicant may either choose to pay additional search fee(s) or choose not to pay any additional search fee.
The applicant may choose all identified inventions for a further search, but may also make a sub-selection. In other words, if the partial search report identifies the presence of n inventions, each corresponding to certain claims, the applicant may choose to pay between 0 and n-1 search fees, depending on which of the claims not searched in the partial search report he selects to have searched.
The applicant may contest the finding of the search division during the substantive examination of the application. If the examiner considers the opinion not justified, the paid additional search fee(s) will be refunded. If the examiner considers the opinion justified the examination will proceed on the basis of the searched invention, or where several inventions have been searched, on the basis of the searched invention(s) and corresponding claims selected by the applicant.
The non-payment of further search fee(s) deprives the applicant of the right to choose with which invention to continue in the application, as he is bound to the searched invention (G2/92).
3) In some situations the examiner may consider that the patent application fails to such an extent to comply with the requirements of the EPC that it is impossible to carry out a meaningful search regarding the state of the art on basis of all or some of the subject-matter claimed (Rule 63(1)). Important requirements include the technical character of the claimed subject-matter (Art. 52(2), (3)), the non-exclusion from patentability (Art. 53), the susceptibility of industrial application (Art. 57), the sufficiency of disclosure (Art. 83), clarity and conciseness of the claims and support of the claims by the description (Art. 84). Here we will present several non-limiting examples where Rule 63 may find application for subject-matter not complying with Art. 83 and 84.
4)
- One example is a broad claim supported by only a limited disclosure covering a small part of the scope of the claim. Here, the requirement underlying the application of Rule 63 would be Art. 83 and 84.
- Another example involves claims lacking conciseness. This may involve for instance a large number of claims or many possibilities within a claim (e.g. by use of many ‘and/or’ clauses) that make burdensome to determine the subject-matter for which protection is sought.
- A further example relates to claims lacking clarity. For instance, the subject-matter is defined using parameter(s) that cannot be compared with the prior-art, because the prior-art does not employ the same parameter or employs no parameter at all.
- Still further examples involve claims in divisional applications extending beyond the content of the earlier application as filed.
In reply to the invitation under Rule 63 the applicant may react in three different ways:
- The applicant may simply indicate the subject-matter to be searched and explain why a meaningful search is possible for that subject-matter;
- The applicant may dispute the findings of the examiner. In this case the applicant must provide arguments why a meaningful search is possible. If the examiner is convinced by the applicant’s arguments, the examiner will issue a full search report. If the examiner is not convinced or only partly convinced, the examiner will determine which subject- matter can be searched and will issue a partial search report for that subject-matter. In exceptional situations the examiner may issue a declaration that no meaningful search is possible. The applicant may, as a main request, file arguments against the findings of the examiner, and as an auxiliary request, indicate subject-matter to be searched.
- If the applicant decides not to reply to the invitation, the examiner will determine what to search.
As indicated above, based on the applicant’s reply the examiner may issue a full search report, a partial search report or in exceptional situations a declaration that no search could be carried out.
Where the examiner has drawn up a partial search report, during the substantive examination the Examining Division will invite the applicant to restrict the claims to the subject-matter searched, unless it finds that the invitation was not justified (Rule 63(3)). If in response the applicant contests the restricted search and the Examining Division is convinced by the applicant’s arguments, an additional search will be carried out at the examination stage.
In the exceptional cases where the examiner has issued a declaration that no meaningful search is possible, the application will not contain searched subject-matter. Following the text of Rule 63(3) “invite the applicant to restrict the claims to the subject-matter searched” the applicant cannot maintain any claim, as no subject-matter has been searched, leading to a refusal of the application. A possibility to continue with the invention is to file a divisional application in which claims have been formulated that the search division will deem searchable.
5) In some situations the examiner may issue a communication under Rule 62a(1) and Rule 63. In response the applicant may indicate the claims that fulfill the requirements of Rule 43(2) and the subject-matter to be searched and explain why a meaningful search is possible. The subject-matter of both indications must correspond to each other. The applicant may also challenge the findings of the examiner by submitting a reasoned reply to both invitations. Further, the applicant may reply to only one of the invitations or to none. In this case the examiner decides what to search.
6) If in addition to an invitation under Rule 62a(1) and/or Rule 63 a non- unity objections is raised the applicant’s reply may deal only with the first invention identified by the examiner.
7) Another reason for excluding a subject-matter from the search is non- payment of claims fees. The search does not include claims above the number of fifteen for which no additional fee has been paid (Rule 45(1) and (3), Rule 162(1) and (4)).
Hence, based on the above considerations the examiner will issue a full search report, a partial search report, or in exceptional cases a declaration that no meaningful search is possible.
According to Rule 61(1) the search report mentions those documents, available to the EPO at the time of drawing up the report, which may be taken into consideration in deciding whether the invention to which the patent application relates is new and involves an inventive step. The search report classifies the documents according to the following categories: X: particularly relevant document when taken alone; Y: particularly relevant document when taken into combination with another document classified as Y; A: document defining the general state of the art, O: non-written disclosure; P: intermediate document published in the priority interval or on the date of priority, T: document published after the filing or priority date and cited for the principle or theory underlying the invention, E: potentially conflicting patent document (prior right), D: document cited in the application and L: document throwing doubt on a priority claim, establishing the publication date of another citation or relevant for double patenting.
Apart from these documents the search report could also mention claims or group of claims that were not included in the search for the reasons discussed above.
When the search is conducted less than 18 months after the filing date it will not be possible at the time of the search to completely search for potentially conflicting European and international patent applications within the meaning of Art. 54(3),
i.e. patent applications that were not published before the priority/filing date of the application under search, but which themselves have an earlier priority/filing date and could be relevant for novelty. If applicable, this search will be finished during substantive examination by the Examining Division.
2.2 Search Opinion
According to Rule 62(1) the search report is accompanied by an opinion on whether or not the application and the invention to which it relates meet the requirements of the EPC. In other words, the opinion will list specific objections that the search examiner has, or it will indicate that all requirements of the EPC appear to be met and that a communication under Rule 71(1) or (3) can be expected once the examination stage is entered.
The search report and the search opinion together constitute the Extended European Search Report (EESR).
The search opinion is a mandatory non-binding opinion. The applicant has the possibility to avoid the issuance of the search opinion in cases when the examination fee was paid before the search report is transmitted to the applicant and the applicant waived the right to the communication pursuant to Rule 70(2). In this case, the examination starts immediately after completion of the search report.
The search opinion is not published together with the search report (Rule 62(2)), but is available to the public by way of file inspection after publication of the patent application.
The search opinion may contain reasoned objections tothe patent application. This is the so called negative search opinion. These objections may relate to substantive matters, for example objections that the subject-matter is not patentable, and/or relate to formal matters, for example that some claims are not clear.
In the situations discussed above in which the examiner has excluded subject- matter from the search, the search opinion also invites the applicant to limit the claimed subject-matter on the searched subject-matter.
If the examiner is of the opinion that the application and the invention to which it relates satisfy the requirements of the EPC the examiner will issue a positive search opinion that simply contains a statement giving a positive general opinion. However, if it was not possible to carry out a complete search on potentially conflicting European and international patent applications falling under Art. 54(3), a top-up search will be carried by the Examining Division as discussed above. This will be indicated in the search opinion.
The examiner may issue a positive search opinion even for cases where minor amendments of the application documents may be needed in order for the application to proceed toward a grant of a European patent. In such situations the Examining Division may carry out the necessary amendments in the text proposed for grant appended to the communication under Rule 71(3).
3. Supplementary European Searches
According to Art. 153(7) a supplementary European search report is drawn up in respect of Euro-PCT applications as indicated in the Introduction above. However, for international applications entering the European phase, no supplementary European Search Report is drawn up for the cases where the EPO was the International Searching Authority of the Supplementary International Searching Authority. Application documents on which the search is based are the documents as specified by the applicant when the application enters the European phase. Alternatively, if the applicant amended the application documents within the non-extendible time period of six-months from notification of communication pursuant to Rule 161(1), the amended application documents serve as basis for the supplementary European search.
The supplementary European Search Report is accompanied by the search opinion. In general the supplementary European Search Report has the same form as the Extended European Search Report. However, in the case of a supplementary European Search Report it is also permissible to have no documents cited in the supplementary European Search Report. In such case the expression “No further relevant documents disclosed” appears in the search report. In such cases, the search opinion gives an opinion on patentability of the invention in view of the state of the art cited in the International Search Report.
As from November 1, 2014 when the amended Rule 164 entered into the force, when a problem of lack of unity of the invention arises, the supplementary European Search Report is drawn up on the invention or group of inventions first mentioned in the claims. Together with the partial search report the applicant receives an invitation to pay further search fees for every invention other than the one first mentioned in the claims (Rule 164(1)). In response to this invitation the applicant follows the same procedure as described above in case of direct European applications. Other EPC provisions, for example Rule 62a and Rule 63 discussed above are also applicable in the procedure under the amended Rule 164 (1).
The amended Rule 164 applies to all Euro-PCT application for which the supplementary European search report has not been drawn up by November 1, 2014.
4. Response to the Extended European Search Report
According to Rule 70a(1), in the opinion accompanying the European search report, the EPO shall give the applicant the opportunity to comment on the extended European search report and, where appropriate, invite him to correct any deficiencies noted in the opinion accompanying the European search report and to amend the description, claims and drawings within the period referred to in Rule 70, paragraph 1 i.e. within six months from publication of the search report. Within the same period the applicant must file the request for examination.
According to the text of Rule 70a(1) “where appropriate” the EPO invites the applicant to correct any deficiencies noted in the opinion. This applies to cases where the opinion is negative and there are deficiencies that the applicant must correct. In this case the response to the invitation under Rule 70a(1) is mandatory. In response the applicant may contest the negative findings of the examiner by providing an explanation for the reasons why the applicant does not agree with the examiner’s objections. The applicant may also respond by submitting amended claims and/or amended description in view of the raised objections. In this case the applicant should indicate what has been changed and indicate the basis for the changes in the original application documents. In addition, the applicant may also submit voluntary amendments in accordance with Rule 137(2).
When, as discussed above, the opinion is positive because there are no deficiencies that should be corrected, or there are only minor deficiencies that the Examining Division will correct in a communication under Rule 71(3), the EPO does not issue an invitation. The applicant may still file amendments on his own volition under Rule 137 (2) and correct errors under Rule 139.
However, if the applicant filed the request for examination before the search report and the search opinion were communicated to him, the period to respond to the opinion and/or to file any voluntary amendments is equal to the period within which the applicant must indicate that he wishes to proceed further with the application. This period is communicated to the applicant in a communication under Rule 70a(2). The Guidelines for Examination specify a period of six-months from the publication of the search report.
For Euro-PCT applications subject to a preparation of the supplementary European Search Report as discussed above, the EPO will also invite the applicant to respond to the search opinion within the period for confirming to continue with the prosecution. The Guidelines for Examination specify a period of six months of notification of the supplementary European search report.
If the applicant does not respond to the invitation pursuant to Rule 70a(1) or 70a(2) within the communicated time period, the application is deemed to be withdrawn. These periods are in general non-extendible. An extension of the time limit (beyond the six months) is allowed only in exceptional cases.
V. Substantive Examination
- Introduction
- Formal requirements
- Substantive Requirements
- Official Action - Reply of the Applicant
- Several Sets of Claims
- Final Stage of Examination Proceedings
- Interacting with the Examiner
- Ex parte Oral Proceedings
- Refusal of European Patent Applications
- Appeal Procedure - Interlocutory Revision
- Accelerated Prosecution
- Further Remarks - Reduction of Costs
- Summary
1. Introduction
This part relates to the examination proceedings which are performed after the filing of an application at the EPO and after the issuance of the search opinion. It may be that, at the beginning of the examination proceedings, amendments made by the applicant himself have already been filed, for example in response to the search opinion.
A European Examiner is entrusted with the substantive examination proceedings wherein the Examiner is selected depending on the technical field to which the application relates. The Examiner is a member of the Examining Division, usually consisting of three members.
The purpose of the examination proceedings is to ensure that the application meets the requirements of the European Patent Convention (EPC). The prime task of the Examiner is to deal with the substantive requirements. At the end of the examination procedure the Examining Division will either issue a decision to grant a patent or will refuse the application. The presentation of important aspects of the examination proceedings takes into account the “Guidelines for Examination in the EPO”, especially Parts C and H thereof.
2.Formal requirements
Some formal requirements have to be fulfilled before the Examining Division can start the examination of a European patent application. The applicant has to file a request for examination and he has to pay the official examination fee. According to Rule 70 EPC the request for examination may be filed from the date on which the application is filed up to the end of six months after the date on which the European search report is published in the European Patent Bulletin. Within the same time frame the designation fees also have to be paid.
If the request for examination is not filed, the application is deemed to be withdrawn (Art. 94(2) EPC). Then the applicant has a possibility to file a request for further processing in accordance with Art. 121(1) EPC wherein an official fee has to be paid and the omitted act must be completed within a time limit of two months.
3.Substantive Requirements
According to Art. 52 EPC there are four basic requirements for patentability: (a) there must be an invention belonging to any field of technology, (b) the invention must be susceptible of industrial application, (c) the invention must be new, and
(d) the invention must be based on an inventive step. The invention must also be described in a manner that it can be carried out by a person skilled in the art (Art. 83 EPC). Furthermore, the invention must be defined by means of technical features and must relate to a technical problem and thus define the technical features in order to define the subject-matter for which protection is sought, in the claims.
Art. 52(2) EPC contains a non-exhaustive list of what should not be regarded as inventions. This list includes discoveries, scientific theories, mathematical methods, aesthetic creations, schemes, rules and methods for performing mental acts, playing games or doing business, and programs for computers or presentation of information. Art. 53 EPC further defines exceptions to patentability, namely matter which would be contrary to the “ordre public” or morality. European patents are also neither granted in respect of methods for treatment of the European or animal body by surgery or therapy and diagnostic method practiced on the human or animal body wherein this provision does not apply to products, in particular substances or compositions, for use in any of these methods (Art. 53(c) EPC).
4. Official Action - Reply of the Applicant
As has been pointed out in the previous chapters, an applicant may perform claim amendments in response to the search opinion. At the beginning of the examination procedure, the Examiner must check whether those subsequently filed claims satisfy the requirements of Art. 123(2) EPC, i.e. that they do not contain added subject-matter. If the basis for the amendments is not evident for the Examiner, he may send a communication pursuant to Rule 137(4) EPC to the applicant. It is then applicant’s duty to submit the corresponding information to the Examiner.
At the beginning of the examination proceedings the Examiner studies the application documents. Usually the Examiner has already started studying the application documents because he was responsible for carrying out the search and for submitting to the applicant the search opinion. In such a case he merely concentrates on any reply to the search report and on the amendments that have possibly been filed by the applicant in response to the search opinion.
If the Examiner has any further objections, for example because there are some deficiencies in view of the amendments, or objections as to lack of patentability, unity or sufficiency of disclosure, then he has to issue the communication in accordance with Art. 94(3) EPC. According to Art. 94(3) EPC the Examiner shall invite the applicant, as often as necessary, to file his observations and, subject to Art. 123(1) EPC, to amend the application.
When preparing the communication the Examiner will consider those prior art documents which have been cited in the previous search report, together with any possible amendments as filed by the applicant (i.e. the most recently filed claims). According to Rule 71(2) EPC the communication should contain a reasoned statement which explains all of the requirements of the EPC which are not met. In general, all objections should be raised in the first official action, which, however, is unfortunately not always the case. It may for example happen that the Examiner becomes aware of further prior art documents at a later time during the examination proceedings so that in view of this he may issue a further communication at a later state of the proceedings. It may also happen that in a first communication objections only in regard to e.g. the admissibility of amendments or the scope of the claims are made and that in a further official action objections in regard to lack of patentability e.g. novelty and/or inventive step are raised.
The communication invites the applicant to file his observations and to correct any deficiencies or to file any amendments. Concerning the amendments the applicant has a possibility to perform amendments to the descriptions and/or the claims and/or the drawings. When performing amendments, the applicant should be aware of the requirements of Rule 137 EPC. According to Rule 137(2) EPC the applicant may amend the description, claims and drawings of his own volition in response to communications by the European Patent Office under Rule 70a(1) or (2) EPC or Rules 161/162 EPC. According to Rule 137(3) EPC no further amendment may be made without the consent of the Examining Division. Even though this rule seems to contain a rather strict requirement in regard to the possibility to perform amendments to the application documents, the claims of a European patent application are oftentimes amended several times after the filing date and prior to the grant of a European patent. This is due to the general rule that the Examiner should always balance the interests of the applicant and the procedural efficiency. In view of this it is quite common that an applicant performs amendments to the claims after receipt of the European search report and, if necessary, also after receipt of the first communication pursuant to Art. 94(3) EPC.
The communication also includes a time period for filing the reply. According to Rule 132(2) EPC the time limit is not less than two months and not more than four months. Under certain circumstances it may be up to six months. In general the Examiner sets a four months’ deadline for filing a reply to the objections raised therein. If necessary, the applicant may file a request for extension of said time limit for a further two months so that in such a case the total time limit is six months for the issuance of the communication.
Sometimes it will become necessary for the applicant to ask the Examiner for a further extension of the time limit. This will be granted only very exceptionally and in view of a reasoned statement. Such a reasoned statement may be e.g. that additional experiments are necessary for showing the inventive step.
The failure to reply in due time will cause the application to be deemed withdrawn. Then the applicant has the possibility to file a request for further processing in accordance with Art. 121 EPC.
In its reply, the applicant should deal with each and every objection raised in the communication. Where necessary the claims and/or the description should be amended. If the claims have already been amended, the description should be adapted to the amended claims. The adaption of the description may also be filed at a later time, for example after the Examiner has acknowledged that amended claims fulfil the requirements of the EPC.
In case the communication contained novelty and/or inventive step objections, the applicant should explain the differences between each of the prior art documents cited by the Examiner and the subject-matter of the (possibly amended) claims. The discussion of the inventive step should be based on the problem-solution- approach as adopted by the EPO. Thus, it is necessary to discuss the technical effects resulting from the difference with respect to the closest prior art. Quite often the applicant performs additional experiments, especially in the field of chemistry, in order to show the technical effects with respect to a specified prior art document. The filing of additional experimental data during the substantive examination proceedings is routinely allowed at the EPO. However, new experimental data must be based on those technical effects which are already described in the patent application or which are implied by or at least related to the technical problem initially suggested by the original application text.
When filing amendments, the applicant is required to indicate where basis for the amendments can be found in the original application text. Even combinations of amended features should be based on the application documents as filed. Currently, no handwritten amendments are allowable.
The reply of the applicant should also contain a request to the Examiner to submit a further official action or to have a telephone discussion with the applicant’s representative or even to summon for oral proceedings before the members of the Examining Division. These additional requests serve to avoid any rejection merely on the basis of the written arguments.
The Examiner has to check the letter of reply and the amended documents, if any. It may happen that on the basis of the reply filed by the applicant the Examiner is convinced that a patent should be granted. The Examiner may call the applicant’s European representative if there are only some minor objections to be dealt with. If the Examiner maintains substantive objections he will issue a second communication pursuant to Art. 94(3) EPC which contains a reasoned Statement why he still maintains some or all of the objections previously raised. Sometimes the Examiner raises new objections or refers to new prior art documents.
5. Several Sets of Claims
It may happen that during the examination procedure the applicant files more than one set of amended claims. For example he may file an amended set of claims in accordance with a Main Request and one or more sets of claims in accordance with the one or more Auxiliary Requests. Together with these various sets of claims he should submit the arguments why according to his position each of these sets of claims fulfils the requirements of the EPC.
It may even happen that the applicant files more than one set of claims in view of Art. 139(2) EPC. According to this Article national rights of earlier date can be invoked, after the grant of the European patent, in national proceedings. Where these national rights exist, the applicant has a legitimate interest in submitting separate claims wherein one set of claims is filed in regard to such a national right. The filing of two sets of claims should then be allowed. However, it is the responsibility of the applicant to make the necessary limitation of the scope of the application with respect to the national right. If a separate set of claims is filed during the substantive examination proceedings in view of a national right having an earlier filing date, the Examiner will usually not examine as to whether this second set of claims contains the correct and necessary limitation with respect to this national right. He will only check the admissibility of the amendments in the light of the original disclosure.
6. Final Stage of Examination Proceedings
If the Examiner considers that the claims are allowable and meet the requirements of the EPC, the next step is for the Examiner to check whether the description should be amended. The Examiner himself may perform some amendments to the description to adapt it to the claims. He may even insert a brief reference to the prior art documents cited by him. Alternatively, he may also call the applicant’s European representative by telephone in order to discuss as to which further amendments should be performed. Typical examples of the amendments which may be made by the Examiner himself include the conversion of units into the correct SI units, the correction of some linguistic errors or even the deletions of some vague statements such as “and the like”. He may even insert into the claims some reference numerals.
If the Examiner is convinced that a patent should be granted, the Examining Division must inform the applicant of the text on the basis of which it intends to grant a patent (Rule 71(3) EPC). If amendments are proposed, these must be identified in this text. The communication under Rule 71(3) EPC also includes the invitation to pay the fee for grant and publishing and to file the translations of the claims in the two languages of the EPO other than the language of the proceedings. Thus, if the application was filed in the English language, the claims have to be translated into German and French.
The communication under Rule 71(3) EPC sets a time limit of four months which is not extendable. If the applicant pays the fees and files the translations, this act will be considered as an approval to the text intended for grant (Rule 71(5) EPC). The quality of the translation of the claims will not be checked by the EPO. This is the duty of the applicant.
The communication under Rule 71(3) EPC additionally contains the title of the invention in the three languages of the EPO. The communication also contains the name of the applicant and the international patent classification. In the event that the renewal fee is due between the issuance of the communication under Rule 71(3) EPC and the proposed date of the publication of the mentioning of the grant, the EPO will also indicate that the publication will be performed only after the renewal fee has been paid.
When the applicant receives a communication under Rule 71(3) EPC, it is the applicant’s responsibility to check whether the application documents are complete and whether amendments as proposed by the Examiner are acceptable. The four months’ time limit for paying the grant and publishing fee and for filing the translations of the claims is the last possibility for the applicant to check the application documents.
Accordingly, the communication under Rule 71(3) EPC contains important information and explicitly specifies the text on the basis of which a patent should be granted.
7. Interacting with the Examiner
The examination of a European application does not involve a mere negotiation with the Examiner regarding patentability. It is necessary to engage proactively with the Examiner. Each objection raised in the official actions, must be addressed in a reasonable manner. It is, of course, legitimate to argue that objections are not justified. However, success is unlikely if it is attempted to overcome objections using superficial arguments or inappropriate claim amendments. Amendments must respond to the specific objections that have been raised, and arguments should be explained in a comprehensive manner explaining either why it is considered that the objections are not justified or why it is felt that the objections have been overcome by amendment or, for instance, by the filing of supplementary experimental evidence.
Examiners often require supplementary experimental evidence in order to prove that claims involve an inventive step over their entire scope. The experimental evidence must be appropriate in that it must represent a fair comparison with the closest prior art and enable generalization of the demonstrated advantage over the entire scope of the claims.
EPO prosecution is primarily a written procedure. It is unusual to have a face-to-face interview with an Examiner, unless the Examiner agrees that an interview may be useful in order to resolve confusion regarding the Examiner’s or applicant’s arguments. Rather than a face-to-face interview, a telephone conversation may be sufficient for this purpose. A telephone conversation may also be helpful in order to resolve minor issues such as when the Examiner considers that the claims are allowable and all that is required is adaption of the description.
Interviews and telephone conversations are not formal procedures since any agreement reached with the Examiner is subject to the views of the other two members of the Examining Division. For this reason, Examiners are often reluctant to discuss substantive issues in such a telephone conversation. Submitting written arguments regarding substantive issues is in any case typically the best way to ensure that the Examiners understand applicant’s arguments. Thus, in general, EPO prosecution differs from the prosecution before other patent offices in that there is a limited scope for interaction with the Examiner other than in writing.
8. Ex parte Oral Proceedings
If deficiencies persist in an application after the applicant has responded to an official action, it is open to the Examiner to consult with the other members of the Examining Division. If there is agreement, he may issue a decision of refusal, or, if previously requested by the applicant, issue a summons to oral proceedings. If the applicant has requested oral proceedings as an auxiliary measure, the application cannot be refused until oral proceedings have been held. Failure to appoint oral proceedings following a request is a fundamental procedural violation and any decision taken becomes null and void.
It is difficult to predict when a summons to oral proceedings will be issued in place of a further examination report. This is highly dependent on the preferences of the Examiner. Certain Examiners are content to keep issuing official actions until all objections are deemed to have been overcome. At the other extreme, if oral proceedings have been requested, some Examiners are all too happy to issue a summons following the applicant’s response to the first official action. From the EPO’s perspective, this practice is in line with the overriding principle that the final decision, i.e. grant or refusal, should be taken after as few steps as possible and indeed, some practitioners have reported an increased tendency of Examiners to issue a summons rather than a second examination report. The issuance of a summons forces the applicant to carefully consider its position in the face of a notification of intended refusal. If a serious attempt has been made to overcome the objections raised in the first examination report but a summons has been issued nonetheless, it may be difficult to succeed at oral proceedings without amending the claims or submitting new arguments or evidence. Sometimes it is possible to convince the Examining Division to change its mind when presenting arguments orally in a face-to-face manner. The two members of the Examining Division other than the primary Examiner will likely have had little contact with the case prior to the oral proceedings, so it may be possible to convince these members of the merits of the application by engaging with them in person.
In response to the summons and prior to the oral proceedings, it is possible to file amendments and written arguments. At this stage, it is advisable to file one or more auxiliary claim sets in case that the Examining Division maintains its objections against the main claim set. If the Examining Division takes the view that an auxiliary claim set is allowable, it will communicate this to the applicant before the oral proceedings and give the applicant the opportunity to withdraw higher ranking claim sets so that the oral proceedings can be cancelled.
9. Refusal of European Patent Applications
If, despite the applicant’s arguments or amendments of the claims or the filing of evidence, the Examining Division maintains the objections, the application will be refused. A refusal may even be made at the end of the oral proceedings before the Examining Division. Then the Examining Division issues a written decision which must be based on the grounds on which the application is refused. It may only be based on the grounds or evidence on which the applicant has had an opportunity to comment (right to be heard) (Art. 113(1) EPC). The written decision must contain the substantiation why the patent cannot be granted.
10. Appeal Procedure - Interlocutory Revision
The applicant who is adversely affected by the decision to refuse the application has a right to appeal (Art. 107 EPC). The appeal has a suspensive effect. The notice of appeal has to be filed within two months of notification of the written decision. Together with the notice of appeal, an official fee for the appeal has to be paid (Art. 108 EPC). Within four months of notification of the written decision, the statement setting out the grounds for the appeal has to be filed (Art. 108 EPC).
If the Examining Division considers the appeal to be admissible and well-founded, it shall rectify its decision (Art. 109(1) EPC). If the appeal is not allowed within three months of receipt of the statement of grounds, it shall be remitted to the Board of Appeal without delay, and without comment as to its merit (Art. 109(2) EPC).
Thus, the Examiner has to consider the reasons for the appeal. It is therefore recommendable to file any further amended sets of claims (Main Request and optionally one or more Auxiliary Requests) in order to convince the Examining Division that the claims of one of these sets of claims are allowable. Together with the grounds for the appeal the applicant may even file additional experimental data in favor of the inventive step and in view of the closest prior art.
If the Examining Division takes the stand that on the basis of either one of the above-mentioned sets of claims a patent should be granted, the reasons for the appeal will not be remitted to the Board of Appeal. Then the Examining Division will send a further communication to the applicant. If, together with the reasons for the appeal the applicant filed amended claims and an amended description, and if the Examining Division rectifies its decision, the next communication will be the communication under Rule 71(3) EPC.
If the Examining Division does not rectify its decision, the appeal procedure before the Board of Appeal will start. Concerning this procedure reference is made to the chapter “Appeal procedure” in this book.
11. Accelerated Prosecution
The backlog of unexamined European applications has increased in recent years. It is not uncommon for applicants to wait several years for the examiner’s reaction to a submission filed in response to objections. The EPO is aware of the growing backlog. There are several cost-free measures available for accelerating the prosecution of an application should there be a desire to obtain a patent quickly.
11.1 PACE
Applicants may request accelerated search and examination under the PACE programme. A request for accelerated search is not necessary for European applications which do not have a claim to priority; in such cases, the EPO issues the extended search report within six months of the filing date. For applications which do claim priority, the EPO only indicates that it will make every effort to issue the extended search report “as soon as possible”. In the case of a request for accelerated examination, the EPO aims to issue a first examination communication within three months of receipt of the applicant’s response to the search opinion or, in the case of Euro-PCT applications for which the EPO was the International Searching Authority, within three months of the response to the Rule 161 Communication, provided that the PACE request is filed with the response. The three month period also applies to subsequent examination communications. It is, however, important for applicants to cooperate by responding within the initial term set by the examining division. Requests for term extensions will likely cause a loss of PACE status.
11.2 Patent Prosecution Highway (PPH)
The PPH consists of a number of bilateral and trilateral agreements between the world’s major patent offices, including the EPO, USPTO, JPO, UKIPO and KIPO. The aim of the PPH is to reduce the burden on examiners by allowing them to make use of work done by other patent offices in respect of equivalent applications. It is certainly possible to use PPH in order to accelerate the prosecution of a European application. However, this is not a given. The differences between the practices of the various patent offices inevitably mean that the conclusions of foreign examiners may not be accepted by EPO examiners.
In order to be eligible for participation in the PPH, examination of the European application must not have commenced. Moreover, it is necessary for all of the claims of the European application to have the same scope or a narrower scope than the allowed claims of the reference application. The European application must not contain additional independent claims beyond those contained in the reference application. A declaration to this effect must be filed with the PPH request. Additional requirements are the filing of copies of all of the office actions issued in respect of the reference application, copies of the non-patent documents cited in these office actions, a copy of the claims of the reference application that have been deemed allowable and, for example, in the case of a Japanese reference application, translations of the office actions and claims.
It is not necessary for all of the claims of the reference application to have been deemed allowable. A PPH request can be filed on the basis of a reference application for which a positive opinion has been issued in respect of only some of the claims.
The PPH may be an effective way to accelerate the prosecution of a European application. However, as a note of caution, it should be remembered that the EPO has unique requirements as regards patentability. An amended claim accepted by, for example, a US or JP examiner may be found by the EPO examiner to add subject-matter extending beyond the content of the application as originally filed. It is also possible that the claims of a European application are prejudiced by a post-published European application which is not citable against the reference application. Conversely, the limitations of the claims in the reference application may not be necessary in order to overcome objections raised by the EPO examiner. The pros and cons of a request for PPH should therefore be considered on a case- by-case basis. A request for PPH may do nothing more than unnecessarily limit the scope of the claims of a European application without dealing with the EPO examiner’s objections.
12. Further Remarks - Reduction of Costs
Obtaining a European patent is not a cheap exercise. Aside from representative’s fees, there are a number of official fees, some of which are variable. Being aware of these fees can help to reduce the overall costs of prosecution. Official fees can be minimized in the following ways:
- File the application with a maximum of 15 claims in order to avoid excess claim fees (as of March 2017: € 235 for the 16th to the 50th claims and € 585 for the 51st and each subsequent claims.
- Reduce the number of pages of the specification so as to reduce or avoid the excess page fees.
- File applications online - this provides a saving of costs versus other forms of filing.
- Avoid unnecessary penalty fees such as the 50% surcharge for late payment of a renewal fee and the fee for further processing.
13. Summary
Patent prosecution before the EPO is constantly developing. The developments are driven by the EPO’s desire to improve the efficiency of prosecution as well as an increased cooperation with other patent offices. Despite the best intentions of the EPO, certain changes have created pitfalls for the unwary. It is therefore essential to have an understanding of the impact of the changes as and when they are made in order to make the best use of the European prosecution procedure. Clear communication between applicants and their representatives is also important for an effective prosecution process and obtaining optimal protection.
VI. Amendments
- Introduction
- Art. 123(2) EPC: Prohibition of Added Matter
- How to compare information content?
- Art. 123(3) EPC and its Interplay with Art. 123(2) EPC
- Art. 123(3) Numerical Ranges
- Art. 123(3) Feature Combinations
- Art. 123(3) Intermediate Generalizations
- Disclaimers
- Rule 139 EPC and Corrections of Errors
- Conclusion
1. Introduction
In all of the major patent systems of the world, an applicant may amend his patent application (and in particular the claims) after the patent application has been filed. This can be done e.g. to take into account objections which have been raised by the responsible patent office. It is also universally true that a patent applicant is restricted to some extent in the amendments he can make. This is also true for proceedings at the EPO.
Generally, it is considered that there is a public policy reason for restricting an applicant’s possibility to amend. Firstly, because the state of the art is defined based on the filing date of the application in question, it makes sense that only inventions which were actually disclosed by the applicant on the filing date can be protected in that application. Secondly, it is considered that any third party should be able to obtain a copy of a published patent application belonging to their competitors, and reasonably be able to predict which claims may be granted. This is only possible if amendment options are restricted.
Compared to other jurisdictions, the EPO is quite restrictive in terms of allowability of amendments, and in particular in the context of assessing whether a given amended claim finds adequate support in the original application text. When an applicant at the EPO asks to make an amendment, it is the applicant that has the burden of proof in showing that the amended claim finds a proper basis in the original application text. Furthermore, the standard of proof needed is a high one; although the EPO typically only requires proof on the basis of the “balance of probability”, there are some specific cases where a point has to be proven “beyond reasonable doubt”. This much higher standard of proof is applied by the EPO in the context of basis for amendments, as is clear from a long line of Case Law stretching back to the 1980s.
When comparing three major jurisdictions such as the EPO, the JPO and the USPTO, it is generally considered that the EPO takes the toughest view on allowability of amendments followed by Japan and then followed by the USPTO.
2. Art. 123(2) EPC: Prohibition of Added Matter
The most important article of the EPC from the perspective of allowability of amendments is Art. 123(2) EPC, which prohibits amendments which add new matter. This EPC article applies to amendments made at any time, i.e. both during prosecution and in post-grant proceedings such as opposition cases. Art 123(2) EPC is not immediately concerned with the scope of the claims, but rather with their information content. This means that both a narrowing amendment and a broadening amendment can cause non-compliance with Art. 123(2) EPC; in practice, it is often narrowing amendments which result in added matter problems.
In order to test whether a given amendment adds matter or not, it is necessary to compare the information content of the amended claim with the information content of the application as filed. If the amended claim includes no new information, then it complies with Art. 123(2) EPC.
For the purposes of this comparison, it is of course necessary to know what the application as filed is. It has been established through Case Law that the application as filed includes the description, claims and drawings (see G 3/89). If the European patent application is a normal European patent application, it is the documents which are filed with the EPO which are the relevant ones. If priority is claimed from an earlier application, e.g. an earlier US provisional application, then the priority document cannot be taken into account (see T 260/85). If the application is a PCT application, e.g. filed in Japanese and later entered into the EP Regional Phase with the filing of a translation, the application as filed would be the original Japanese-language PCT application (see Art. 70(2) EPC). Specifically, the translation filed on entry into the EP Regional Phase does not count as the application as filed in such a situation. Also, the abstract is never part of the application as filed for the purposes of assessing added matter (see T 246/86).
3. How to compare information content?
Unfortunately, there is no simple test which can be applied to give a conclusive answer as to whether or not a given amendment complies with Art. 123(2) EPC. Instead, this must be assessed on a case-by-case basis bearing in mind the prevailing Case Law.
3.1 The Novelty Test
There has been one attempt at developing a model which allows an easy prediction of whether or not an amendment is allowable. This is called the “novelty test”. Unfortunately, the novelty test is of limited use. When applied, it can give a positive or negative result. A negative result means that it can be said, with certainty, that the amendment in question is not allowable. However, a positive result is not so clear; this only means that the amendment could be allowable, but it could also be unallowable. Thus, this test can be used to prove that a given amendment is not acceptable, but it can never be used to prove that an amendment is in accordance with the law.
The novelty test is relatively simple to apply. One simply takes the amended claim and compares itwiththe application as filed, pretending thatthe entire application as filed is a prior art document. One then examines whether the amended claim is novel over the “pretend prior art document”. If the result of the analysis is that the claim is novel, then this is a clear indication that the amendment in question is not allowable. If the claim is not novel, no clear conclusion is possible.
3.2 The Principle of Direct and Unambiguous Derivability
Because the novelty test does not provide a conclusive answer, there are situations where further analysis is necessary in order to conclusively find out whether a given amendment is acceptable or not. The admissibility of an amendment is to be decided solely on the basis of an examination of whether the amendment is directly and unambiguously derivable from the application as filed (see T 288/92).
The standard of “direct and unambiguous derivability” is therefore the currently applied standard. There is a significant body of Case Law which explains how this is to be assessed in certain specific situations: sections 4-6 of this chapter analyze some of this Case Law.
Generally speaking, the EPO does not have a strict requirement that any wording used in an amended claim must be found in the application as filed. However, experience shows that EPO Examiners are extremely skeptical if any new words (i.e. words not present anywhere in the application as filed) are used in an amended claim; this will invite high levels of scrutiny and it is almost certain that an explanation will be required why this has been done. It is therefore generally safer to only use wording which is found in the application as filed. Even when relying only on the original wording, there is no guarantee that an amendment will be allowed because the EPO is very interested in examining whether a change of context of the original wording has been made, which change of context might add new information. Therefore, although this is in itself not enough to be safe from the strict added matter assessment, it is a good start.
4. Art. 123(3) EPC and its Interplay with Art. 123(2) EPC
Art. 123(3) EPC applies only to post-grant amendments, e.g. amendments during opposition proceedings. While Art. 123(2) EPC is about the information content of claims, Art. 123(3) EPC deals with something else, namely the scope of the claims. Art. 123(3) EPC basically says that, after grant, a claim may not be amended so as to extend the scope of protection relative to the granted claims. This is assessed in a rather simple way; if there is an embodiment which falls within the amended claim, but which is not covered by any granted claim, then the scope of protection has been extended.
One important characteristic of European Patent Law results from the interplay between the two parts of Art. 123 EPC, when taken in conjunction with the fact that non-compliance with Art. 123(2) EPC is a ground of opposition. The issue is best explained by way of a simple example:
Imagine that a European patent application is filed with a relatively broad claim. The Examiner raises an objection of lack of patentability, to which the applicant responds by amending the claim to introduce several restricting features. The Examiner accepts the amendment and grants a patent based on the amended claims which were filed. If the patent is now opposed, an opponent can argue that the patent is invalid because of the amendment made. Although it was accepted by the Examiner, if an opponent can convincingly argue that the amendment in fact does not comply with the requirements of Art. 123(2) EPC, then the patent will be revoked unless the patentee can further amend the claims in order to fix the problem by further amendment. This is where the interplay with Art. 123(3) EPC arises; as explained above, it is not possible to extend the scope of protection after grant, for which reason it would not be possible during opposition to remove a limiting feature which had been introduced into the claim before grant. Therefore, if an amendment made during prosecution is in fact not allowable (despite the examiner believing it is acceptable), this will render the granted patent vulnerable to revocation unless there are narrower fallback positions available which have an adequate basis in the application as filed. It is because of this interplay between the two parts of Art. 123 EPC, and because added matter is a ground of revocation in Europe, that European patent attorneys are often very careful about restricting amendments made during prosecution. Because experience has shown that valuable patents can be revoked for added matter, even when an examiner has approved the amendment in question, European patent attorneys often prefer to advise cautiously on amendment matters in order to provide the best advice for their clients and in order to ensure that the granted patents are not vulnerable to formal attacks.
5. Numerical Ranges
One type of amendment which often comes up in all technical fields is the restriction of a broad numerical range to a narrower one. Generally speaking, when an originally filed claim indicates a broad range, the applicant is not free to narrow this as he sees fit; narrowing of the range is only possible if the application as filed provides an adequate basis for the desired narrowing. The ideal situation is that the narrower range which the applicant wants to amend to is expressly disclosed in the application as filed; then the amendment to that disclosed range will be possible. It may also be possible to rely on only one endpoint of a preferred sub-range, and use this in conjunction with the endpoint of the original broader range; for example, if the original claim discloses a range of 2-8% and a preferred range of 4-6% is disclosed, it should be possible to amend the claim to refer to either e.g. 2-6% or e.g. 2-4% (see T 2/81 and T 1170/02). Generally speaking, it is not possible to generate new endpoints for a numerical range from isolated values disclosed, e.g. from individual data points disclosed in a table or graph but not mentioned as being interesting as endpoints of a range.
6. Feature Combinations
As mentioned above, the EPO generally considers that the wording found in an amended claim should ideally be found also in the application as filed in order for the amendment to be allowable. However, there are also many situations where the amended claim only includes originally disclosed words, but the amendment still is not allowable. This can happen e.g. because the EPO believes although the claim includes only originally disclosed features, the original disclosure did not specifically foresee the combination of features recited in the amended claim, so that a new information is generated by the way the features have been combined.
In this context, there is a strong line of Case Law at the EPO relating to the generation of new information by the carrying out of selections from multiple lists. According to this line of Case Law, where the information in the claim can only be arrived at by singling out items from two or more lists in the original disclosure, which items were not specifically highlighted in the application as filed as being preferred, will result in the generation of new matter.
As an example, one could consider the original, general disclosure of the reaction of two chemicals, namely a chemical of type A and a chemical of type B. The original disclosure might mention that the chemical of type A could be the chemical A1, A2, A3, A4 or A5 and that the chemical of type B could be e.g. B1, B2, B3, B4 or B5. If, based on this disclosure, the applicant were to write an amended claim directed to the reaction of A3 with B4, the EPO would very likely consider that Art. 123(2) EPC has not been complied with because two selections would need to be made from two lists in the original disclosure to arrive at the amended claim.
Conversely, it would likely be acceptable to write a claim to the reaction of compound A3 with a compound of type B; this is because this claim could be arrived at only by selecting from one list, whereas the EPO only sees the problem when selections are made from two or more lists.
Also potentially acceptable would be a claim where individual options are not picked, but only a shortening of the original list is carried out; thus, it is possible that it would be allowable to write a claim directed to the reaction between a compound which is any of A1, A2 or A3 with any of compounds B2, B3 or B4. However, an amendment of this type would have to be looked at quite carefully also from the general context to see whether it would be allowable, as this is a borderline area. Reverting to the singling out of individual members from lists, it must be mentioned that although this is generally not allowed when two or more lists are involved, it can be allowable if the application as filed highlights the members of the lists which are the ones selected in the amendment. Such “preselection” or “highlighting” could e.g. arise from these being the only ones used in the examples, these being used in at least one example together, or these members being specifically said to be the single, most preferred members of each list. Thus, depending on the circumstances, it may be possible to make multiple selections from multiple groups.
Although this line of Case Law was developed on the basis of literal lists of items,
e.g. lists of substituents or lists of reagents, its application has been broadened significantly and it can fairly be said to apply whenever multiple options are given for any feature. Thus, the concept of “selection from multiple lists” comes up quite often in practice.
7. Intermediate Generalizations
Another area where the EPO takes a strict approach is when mixing parts of the original disclosure which were originally disclosed at different levels of generality. This commonly comes up when features are taken from an example and used to amend an originally filed claim. This typically would attract an objection from the EPO examiner, as the EPO generally considers that the skilled person, when confronted with the disclosure of a specific example, would assume that the example discloses a “package” of features from which it is not normally allowed to extract individual features and so separate them from the other specific features.
Therefore, it is generally very difficult to amend a pre-existing claim based only on an example without accidentally contravening Art. 123(2) EPC, for which reason this is generally not advisable. If, in a specific situation, this is strictly necessary, it is possible to extract a feature from an example if it can be successfully argued that the features from the example which were not used as limitations in the claim would not be understood by the skilled reader as being essential and are not described as such in the original disclosure, the feature left behind are not indispensable to the function of the claimed item, and the features which are taken and those which are left behind are not strongly linked to one another.
The problem of intermediate generalizations is not only seen when Examples are relied on for amendments; this can apply to any situation where some information from two parts of the disclosure is blended together, especially when some information contained in those parts is left unused.
8. Disclaimers
Claims most often use positive features to define the invention and limit it from the prior art, it is also possible sometimes for an invention to be defined in part by negative features. These are called disclaimers, and rather than defining the features which characterize the invention, they define specifically what the invention is not.
As a mere example, one could consider defining a car by a positive feature (e.g. the car has been made in Japan) and a negative feature (e.g. the car is not red). The claim would then read “A Japanese car, with the proviso that the car is not red”, and the text “…with the proviso that the car is not red” is the disclaimer.
As will be understood, a disclaimer can of course be included in an originally filed claim; for the purpose of this chapter, we will look only at how and when a disclaimer can be introduced into a claim as an amendment.
When considering whether an amendment to introduce a disclaimer is allowable, there are three separate scenarios to consider.
8.1 Scenario 1: The preferred absence of a certain feature.
If the application as filed expressly discloses that a certain feature is preferably absent, then it is typically allowed to introduce a disclaimer which specifically says that the feature in question is not included. For this type of disclaimer, the amendment is treated like any other amendment and so it must simply be examined whether or not the claim including the disclaimer is “directly and unambiguously derivable” from the application as filed.
8.2 Scenario 2: No mention the feature to be disclaimed.
On the face of it one might think that it is not possible to disclaim a feature if that feature was not at all disclosed in the application as filed. However, the introduction ofsuch disclaimers is possible under a very limited set ofcircumstances (see G 1/03).
According to the Enlarged Board of Appeal, a so-called undisclosed disclaimer can be used to restore novelty over a prior art document, but only if that prior art document falls into the so-called Art. 54(3) EPC prior art category. As is explained elsewhere in this book, prior art documents in this category are other European patent applications which have an earlier filing date but a later publication date relative to the claim in question. This particular use of undisclosed disclaimers is perhaps the most frequent one. Nevertheless, disclaimers of this type should be used with caution; if a disclaimer is used incorrectly or worded wrongly, it could add matter and lead to serious validity problems. Accordingly, disclaimers of this type should only be used with caution.
A second situation where an undisclosed disclaimer could be used is to restore novelty over a pre-published prior art document, but only where the anticipation caused by the prior art is a so-called “accidental anticipation”. Subsequent Case Law has defined what is meant by an “accidental anticipation”, and it is clear now that the EPO considers this to be extremely limited. In particular, a prior art document which would be considered by the skilled person (even if it would be considered and immediately rejected, or immediately dismissed as being unhelpful) by definition cannot contain an accidental anticipation. An accidental anticipation can only take place in a document which would not at all have been considered by the skilled person; this is of course very rare in practice, as it would require the prior art document to be in a completely different field.
A third situation where an undisclosed disclaimer could be used is to remove subject-matter which is non-patentable for non-technical reasons; for example, if a claim encompasses both, excluded (methods of treatment) and non-excluded (cosmetic methods), it would be possible to use a disclaimer to make it clear that the claim covers only the cosmetic uses.
When an undisclosed disclaimer is used, there are various conditions that must be complied with. The disclaimer cannot remove more than necessary, which means that if it is used for novelty purposes it must only exclude embodiments which are actually novelty-destroying. The disclaimer cannot be relevant to inventive step, and the resulting claim (including the disclaimer) must be clear.
8.3 Scenario 3: The feature to be disclaimed is only positively disclosed.
It is generally speaking possible to use a positive original disclosure as a basis for a negative disclaimer (see G2/10). Thus, going back to the original disclaimer example, if the application as filed disclosed that it is preferable that the car is red, then it may be possible to expressly disclaim red cars by amendment. The Enlarged Board of Appeal did however say in their decision that a “reality check” is to be performed, and that a disclaimer of this type cannot be used if the subject-matter of the claim, once the disclaimer has been taken into account, is not directly and unambiguously derivable from the original application text. The Case Law is still currently being developed here so that amendments of this type are still controversial.
9. Rule 139 EPC and Correction of Errors
Rule 139 EPC allows for the correction of errors, e.g. linguistic errors, typing errors, and other such mistakes. Such errors may be corrected on request of the applicant, at any time while proceedings are going on at the EPO. If the request for correction relates to the application text, then the correction must be obvious to the skilled reader. This means that the EPO has to be convinced that (i) the skilled person would immediately see that there is a mistake; and (ii) the skilled person would immediately understand that nothing other than the offered correction is what was intended.
This strict test means that corrections are often difficult to carry out at the EPO.
The European Patent Office takes the view that a correction of an error is merely declaratory in nature; in other words, it simply makes it clear to everyone what was originally meant, and as such the correction does not change the meaning of the original erroneous text.
Because corrections are generally difficult to carry out and only have a declaratory nature, there is often not a particularly high value for applicants associated with the correction of these errors, although it can only be assessed on a case-by-case basis whether it is possible and meaningful to correct a given mistake.
In practice, it is rather cumbersome to correct an error after grant unless post- grant opposition proceedings are going on at the EPO. This is because the EPO no longer considers itself to be responsible, so that the correction has to be arranged at each individual national patent office. Therefore, if an applicant notices errors in the application text and would like to correct these, it is advisable to do so before grant.
10. Conclusion
The EPO’s strict approach to the assessment of added matter is a defining characteristic of European Patent Law and is a real difference with respect to e.g. US patent practice. Because added matter is a ground of opposition, it is an issue which should be taken very seriously by applicants; in particular, it is important for applicants to understand that it is ultimately his responsibility to ensure that any amended claim presented to the EPO does not contain added matter. Even if the EPO examiner is prepared to allow a claim, this does not constitute a final decision that the claim does not contain added matter; the amended claim is still open to a legal challenge after grant by an adverse third party. For these reasons, it is extremely important for applicants to obtain careful advice from their European representatives when amending their claims.
To the extent that applicants file PCT applications with the intention of entering the European Regional Phase, it also makes sense to draft these applications bearing in mind some of the amendment difficulties which are often encountered in Europe. In particular, it makes sense to foreshadow strong fallback positions especially by disclosing preferred features in preferred combinations. It also makes sense to look carefully at the examples in the application text, and to consider adding some further description text highlighting key elements or key features of the examples to allow these to be used more conveniently for amendment.
VII. Remedies
1. Area of Application
In case the applicant or proprietor has failed to observe a time limit vis-à-vis the European Patent Office (EPO), the European Patent Convention (EPC) offers the remedies of:
- Further Processing under Article 121 in connection with Rule 135, and of
- Re-establishment of Rights under Article 122 in connection with Rule 136.
The requirements and procedures in connection with these remedies will be summarized and discussed in the following. Further information may be found in the EPO Examination Guidelines (Guidelines E-VII 2).
As a remark, the pendency of an application does not constitute a time limit in the sense of Article 121 or 122 EPC. This means that for the late filing of a divisional application, with the parent application no longer pending, these remedies are not available.
Furthermore, the fixed date of oral proceedings does not represent a time limit, either. Thus, the remedies are not applicable in case the applicant or proprietor failed to attend the oral proceedings.
2. Further Processing
2.1 General Outline
According to Article 121(1), an applicant who fails to observe a time limit vis-à-vis the EPO may request further processing of the European patent application. This implies that the procedure of further processing is only available in examination proceedings.
The general outline of the procedure can be summarized as follows. If the applicant has failed to observe a time limit, the EPO issues a Communication under Rule 112 EPC, notifying the applicant of the loss of rights and the respective legal consequences. The loss of rights is not limited to a lapse (deemed withdrawal) or refusal of the application as a whole, but may also be a merely partial loss of rights (e.g., loss of claims for which no claim fees have been paid).
In reply to this Communication, the applicant may request further processing. The requirements for the request are stipulated in Rule 135 EPC:
- The request has to be filed within a time limit of two months of the Communication.
- A respective fee for further processing has to be paid.
- The omitted act (e.g., filing of a response, payment of fees) has to be completed within the time limit for making the request.
In case the omitted act is the filing of a response to an Official Action, then the completion of the omitted act requires the filing of a substantive response. Merely filing e.g., a request for term extension or a request for oral proceedings does not satisfy the requirements of Rule 135 EPC (see J 16/92).
The fee for further processing is stipulated in Art. 2(12) of the Rules relating to Fees. In case of a late payment of fees, the fee for further processing corresponds to a surcharge of 50% of the relevant fee; otherwise it is a fixed amount of presently 255 EUR (i.e. March 2017).
If the requirements are met, then the EPO grants the request for further processing. The legal effect of the granted request is that the legal consequences of the failure to observe the time limit are deemed not to have ensued (Art. 121(3)).
In practice, the option of further processing may not only be used as a remedy for accidentally missed time limits in examination proceedings, but also as a two- month term extension with costs.
2.2 Exceptions for which no Further Processing is Available
Generally, the procedure of further processing is applicable to most time limits in connection with the patent application, except those laid down in Article 121(4) and Rule 135(2).
The exceptions in Article 121(4) are those which are particularly relevant in view of legal certainty, and include the 12 month priority term (Art. 87(1)), the appeal term (Art. 108) and the time limit for the petition for review (Art. 112a), and, of course, the time limits for requesting further processing itself and for requesting a re-establishment of rights. Re-establishment of rights may be possible for these time limits under certain conditions (except, of course, the time limit for re- establishment of rights itself).
Most exceptions of Rule 135(2) refer to time limits, for which the EPC already provides a separate remedy on its own, so that further processing is not required:
- Rules 6(1) and Rule 36(2): Term for filing the translation of the European patent application or the divisional application, if the application has not been filed in an Official Language (English, French or German); If these time limits are not met, then the EPO will issue an invitation under Rule 58 EPC to file the missing translation within a period of two months.
- Rule 40(3): Term for filing a certified copy of the application documents if the European patent application has been filed by reference to an earlier application according to Rule 40(2). If this limit is not met, then the EPO will issue an invitation under Rule 55 EPC to file the missing translation within a period of two months.
- Rule 51(2)-(5): Terms in connection with renewal fees (as this rule provides own regulations for late paying of renewal fees).
The above-mentioned Rules 55 and 58, as well as Rules 56 and 59 relate to terms for the applicant to correct defects in the application documents and the priority terms upon invitation of the EPO, and thus already represent a remedy by themselves. Therefore, further processing is excluded.
For the time limits of Rules 62a (independent claims of the same category), 63 (incomplete search) 64 (search report in case of non-unity) and 164 (unity issues in connection with PCT applications), further processing is not available, either. Instead of seeking remedy, the applicant may either try to challenge the respective objections (e.g., of non-unity) in examination proceedings or file a divisional application.
Other exceptions, for which no further processing is available, include the time limit of Rule 16(a) (relating to entitlement issues), 31(2) (deposit of biological material) and 112(2) (requesting an appealable decision on a loss of rights).
2.3 Examples
Further processing is available for most time limits in examination proceedings, except for those relating to the formal requirements, which have their own remedies.
In case the applicant files the application in a non-EPO language and fails to observe the term for submitting the translation into an official language of the EPO (i.e. English, German, French), the EPO will issue an Invitation under Rule 58 EPC to file the translation within 2 months from the notification of said Invitation. For a failure to comply with this additional 2-month term of Rule 58, however, further processing is not available.
As regards the entry of the EP regional phase (time limit of 31 months from the priority date, according to Rule 159 EPC), further processing is available.
Turning to the initiation of the examination proceedings, the EPO sets a time limit of 6 months in the Communication under Rules 70 and 70a EPC. If the applicant fails to meet these time limits, further processing is available. However, it has to be noted that two separate time limits may be involved: The time limit of Rule 70 for requesting examination or confirming the maintenance of the application, and the time limit of Rule 70a for addressing the objections raised in the search opinion. If
both are missed, two requests for further processing would be necessary, and for both, a respective fee has to be paid. This means that in case the applicant intends to maintain the application in accordance with Rule 70, but more time is required for elaborating a response to the search report in accordance with Rule 70a, it is advisable to file the confirmation to maintain the application within the term of Rule 70, and request further processing only with respect to the term of Rule 70a.
As apparent from this example, the procedure of further processing may be used not only as a remedy, but also as a kind of response term extension with costs. For instance, in examination proceedings, the EPO may refuse a request for term extension beyond 6 months, so that the application will be deemed to be withdrawn in case no response has been filed. The procedure for further processing thus offers a further two-month term for the response.
3. Re-establishment of Rights
3.1 General Outline
The second, more complex remedy offered by the EPC is the re-establishment of rights pursuant to Article 122 and Rule 136 EPC.
According to Article 122(1) EPC, the remedy is available to both applicants and proprietors, who in spite of all due care required by the circumstances having been taken, were unable to observe a time limit vis-à-vis the European Patent Office, if the non-observance of this time limit has the direct consequence of causing the refusal of the European patent application or of a request, or the deeming of the application to have been withdrawn, or the revocation of the European patent, or the loss of any other right or means of redress.
The time limits set by the Opposition Division, e.g., for filing a response to a notice of opposition, do not have these direct consequences, so that a re-establishment of rights is not possible for these time limits.
As regards the applicable time limits, a re-establishment of rights is only available for those time limits for which no further processing is available (Rule 136(3)). Furthermore, re-establishment of rights is not applicable in case the missed time limit is the time limit for requesting re-establishment of rights itself (Art. 122(4)). As a corollary, this means that if the above-discussed time limit for requesting further processing has been missed, re-establishment of rights is available.
The time limit and the requirements for requesting a re-establishment of rights are stipulated in Rule 136:
- The time limit for the request for re-establishment of rights generally is two months of the removal of the cause of non-compliance with the period, but at the latest within one year of expiry of the unobserved time limit.
- In case the request for re-establishment of rights concerns the priority term (Art. 87(1)) or the time limit for the petition for review (Art. 112a), then the request has to be filed within 2 months of the expiry of the period.
- An official fee has to be paid (Rules relating to Fees, Art. 2(13), presently 635 EUR).
- The omitted act has to be completed; and
- The request has to include the grounds on which it is based and the facts on which it is based. This means, in particular, that it has to be substantiated that the failure to observe the time limit occurred in spite of all due care.
If these requirements are met and the EPO is satisfied that the failure occurred in spite of all due care, the request will be granted, which again has the legal effect that the legal consequences of the failure to observe the time limit are deemed not to have ensued (Art. 122(3)).
3.2 Deposition of Biological Material
Rule 31(2) is exempted from further processing, and is not explicitly mentioned among the exempted time limits for re-establishment of rights. Still, a re- establishment of rights is not possible for Rule 31(2) in view of the requirement of sufficiency of the disclosure.
In summary, Rule 31 requires that a sample of the biological material has been duly deposited no later than the filing date, and the respective time limits of Rule 31(2) are intended for ensuring that the relevant deposit information has been communicated to the EPO before the publication of the application (or earlier in case there exists a right for file inspection).
As explained in the reasons explained in the Notice of the EPO in published in the Official Journal 2010, page 498, compliance with Rule 31 is crucial in connection with sufficiency of the disclosure, which must be established at the filing date and cannot be remedied at a later stage. Section 3.10 of said notice states:
Consequence of the failure to comply with the requirements of Rule 31 EPC, on the contrary, is that the biological material cannot be considered as having been disclosed pursuant to Article 83 EPC by way of reference to the deposit (see Guidelines C-II, 6.3). The information provided for in Rule 31(1)
(c) and, where applicable, (d) EPC may not be submitted after expiry of the time limit set out in Rule 31(2) EPC since the time limit under Rule 31(2) EPC is excluded from further processing by Rule 135(2) EPC and because a lack of disclosure cannot be remedied by way of re-establishment under Article 122 EPC (see G 2/93, op. cit.).
As a result, despite the fact that it is not mentioned in Article 122 or Rule 136, re- establishment of rights for the time limit of 31(2) EPC is not possible in view of the fact that the sufficiency of the disclosure must be established at the filing date.
3.3 Opposition Proceedings
The wording of Article 122 only applies to applicants and proprietors, not to opponents. Thus, for the opponent, no remedy is available in case the opponent failed to observe the opposition term or the opposition appeal term.
However, the Enlarged Board of Appeal has created an exception for the filing of the Grounds for Appeal (G1/86). This was justified, inter alia, by the principle that all parties to proceedings before a court must be accorded the same procedural rights.
3.4 Substantiation of the Request
The substantiation of the request pursuant to Rule 136(2) must conclusively set out all grounds and facts on which the case relies. The factual basis cannot be altered after expiry of the time limit. A request for re-establishment of rights which relies on general statements only and contains no specific facts does not satisfy the requirement for a duly substantiated request (J 15/10).
Specifically, J 15/10 states that the request for re-establishment of rights must set forth the precise cause of non-compliance with the time limit concerned (i.e. a fact or obstacle preventing the required action within the time limit), specify at what time and under which circumstances the cause occurred and was removed, and present the core facts making it possible to consider whether all due care required by the circumstances had been taken to comply with the time limit concerned.
It is not sufficient to file merely general statements without concrete facts identifying the reasons and the chronological sequence of events which lead to the non-compliance (J 19/05). Filing an insufficiently substantiated request and a second submission with further substantiation is only acceptable if the second submission is still within the time limit (T 287/84). While all facts have to be brought forward within the time limit, it may be possible to file evidence for the facts at a later stage (T 324/90).
3.5 Time Limit for the Request
The time limit of Rule 136(1) is generally within two months of the removal of the cause of non-compliance with the period, but at the latest within one year of expiry of the unobserved time limit.
The cause of non-compliance and the removal thereof is a matter of fact which has to be determined in consideration of the merits of the individual case (J 27/90).
Often, the cause of non-compliance involves some error in the carrying out of the party’s intention to comply with the time limit. The party does not then realize that the error has occurred, and that the time limit has not been complied with, until this fact has been brought to his attention (J 29/86). In these cases, the relevant date of removal of the cause of non-compliance is the day, on which said fact is brought to the party’s attention, e.g., by the receipt of a respective Official Communication, so that the person responsible for the application or the patent should have discovered the error.
The date of actual receipt of the communication is of relevance, not the date of the deemed notification, e.g., pursuant to Rule 126(2) EPC (i.e., date of the communication plus 10 days) (J 7/82).
The relevant date is usually the date on which the communication is received by the responsible person, who generally is either the applicant himself or the authorized representative. If the applicant instructs the authorized representative not to pass on any further communication from the EPO (here: to a computerized service firm for renewal fee payment), then the applicant cannot rely on the fact that information notified to the authorized representative and necessary for continuing the proceedings was lacking (J 27/90).
Under exceptional circumstances, the cause for non-compliance with a time limit may be constituted by financial difficulties (J 22/88).
3.6 Due Care
The condition of “all due care required by the circumstances” has been subject to numerous decisions of the Boards of Appeal of the EPO. Generally, it is necessary to consider the specific circumstances and the merits of each case (T 287/84). Pursuant to T 30/90, “all due care” means the standard of care that the notional reasonably competent patentee, applicant or representative would employ in the relevant circumstances. According to T 1289/10, this also takes into account the known problems associated with the procedure and known solutions how to avoid them.
For the normal case where the cause of non-compliance with a time limit involves some error in the carrying out of the party’s intention to comply with the time limit, due care is considered to have been taken if non-compliance with the time limit results either from exceptional circumstances or from an isolated mistake within a normally satisfactory monitoring system (J 2/86, J 3/86).
Exceptional circumstances may include force majeure, unforeseeable errors in the course of organizational upheaval or restructuring, sudden illness, and others. Generally, the exceptional circumstances need to be explained, and it has to be substantiated why these circumstances lead to an accidental failure to comply with the time limit.
For substantiating that there was an isolated mistake in an normally satisfactory monitoring system, it firstly is required to plausibly show that the system for monitoring time limits existed at the relevant time, normally operated reliably, and included appropriate safety measures, cross-checks, etc., in order to comply with the requirements.
The criterion of due care has to be met by the responsible person, which is the applicant or the appointed representative. If the responsible person entrusts tasks to an assistant (e.g., an employee), and the error was caused by the assistant, the responsible person has to establish that the assistant was reasonably selected, instructed and supervised.
PART C Procedures after Grant
I. Opposition Procedure
- Introduction
- Formal Requirements
- Grounds for Oppositon
- Written Proceedings
- Oral Proceedings
- Amendments
- Intervention of the Assumed Infringer
- Conclusion of the Opposition Procedure
1. Introduction
The European Patent Convention (EPC) provides for a possibility for a third party to challenge a European patent after grant by means of filing an opposition. The proceedings take place before certain institutions within the EPO - the Opposition Division for the first instance, and the Board of Appeal for the (final) second or appeal instance.
In this chapter, an attempt is made to cast some light on the basic concept underlying the EP Opposition and its legal framework, in terms of the procedural aspects as well as the grounds on which an opposition can be based.
2. Formal Requirements
The basic provision governing the Opposition procedure under the EPC is Art. 99. According to Art. 99(1) EPC within nine months from the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. Notice of opposition shall not be deemed to have been filed until the opposition fee has been paid.
Oppositions can therefore only be filed for a limited period of time, namely, for a period of nine months from the mention of the grant of the patent. Oppositions filed earlier will enter into the EPO’s respective file, but they will not be treated as an opposition. They are considered to constitute third party observations under Article 115 EPC (Guidelines for the Examination in the EPO D-IV- 1.1). Oppositions which are filed later will be rejected as being inadmissible under Rule 77 (1) EPC. There is no way to extend the opposition period, and there is also no possibility of re-establishment of rights. The same is true for the payment of the opposition fee.
One aspect of Article 99 EPC is the fact that “any person“ may file an Opposition. While in the early days of the EPC this was construed to also include the patentee, G 9/93 stipulated that the patentee is not “any person“ and, thus, may not oppose his own patent. Patentees can request voluntary limitations or revocations using the new centralized European limitation procedure according to Art 105a, which was introduced with the revision of the EPC (‘EPC2000’). Alternatively, voluntary limitations can be requested separately before the national patent offices of each and every designated state. A notice of opposition may also be filed jointly by more than one person. In such case the opposition is admissible on the payment of only one opposition fee (G 3/99). Oppositions can also be inherited or succeeded to as a part of an overall succession in law.
The formulation “any person“, however, has also been the focus of much debate for another reason. Many times opponents do not want to unveil their identity to patentee for business reasons. For many years in the past, this has been virtually impossible as the EPO considered oppositions filed by a“manofstraw“, i.e. someone who has no interest in the Opposition and merely acts to conceal the identity of an interested party, to be inadmissible. In a later decision (G 4/97) however, the EPO moved away from this practice. Following this decision, oppositions filed by a “man of straw“ are no longer generally inadmissible unless they constitute an abusive circumvention of the law (e.g., when the man of straw acts on behalf of the patentee).
Rule 76 EPC sets forth a number of requirements which are of further importance. This rule requires inter alia that the notice of opposition comprises a statement of the extent to which the European patent is opposed and of the grounds on which the opposition is based, as well as an indication of the facts and evidence presented in support of these grounds. These requirements define the framework within which the opposition will be examined. If the opponent limits the extent to which the European patent is opposed, the Opposition Division has no power to decide on subject matter not opposed (G 9/91 and G 10/91). According to Rule 81, the Opposition Division shall examine those grounds for opposition which are invoked in the opponent’s statement of grounds in the notice of opposition. Grounds for opposition not invoked by the opponent may be examined by the Opposition Division of its own motion if they would prima facie seem to prejudice the maintenance of the patent (G 10/91).
3. Grounds for Opposition
The grounds on which an Opposition under the EPC may be based are set forth in Article 100 EPC, which reads:
This list of grounds for opposition under the EPC is exhaustive. Other grounds, such as an alleged lack of clarity (T 23/86, T 792/95) (Art. 84), or lack of unity (G 1/91) (Art. 82) are not valid grounds for filing an opposition under the EPC, even though clarity of the claims and unity of the inventions are mandatory requirements for the grant of a patent. However, amendments filed during opposition proceedings must comply with the clarity requirement (but not with the requirement of unity of invention). In a recent decision (G 03/14) the Enlarged Board of Appeal concluded that in considering whether for the purposes of Art. 101 (3), a patent as amended meets the requirements of the EPC, the claims of the patent may be examined for compliance with the requirements of Art. 84 only when, and then only to the extent that the amendment introduces non-compliance with Art. 84.
Also, unlawful usurpation is not a valid ground in proceedings before the EPO, but it is, e.g. in national German proceedings.The ground of Opposition under Art. 100(a) includes the “classic“ requirements for patent rights as laid down in Articles 52 to 57 EPC.
While the grounds of Articles 52, 53 and 57 EPC are not frequently used, even though they are highly important in certain technological areas, the gist of the EP oppositions is based on an alleged lack of novelty and/or a lack of inventive step. Although looking at Art. 100(a) it seems that there is only one ground of opposition, in G7/95 the Enlarged Board of Appeal came to the opinion that Art. 100(a) embraces many grounds of opposition and that lack of novelty is indeed a different ground from lack of inventive step. Specifically, the Enlarged Board of Appeal in its decision defined a ground as an individual legal basis for objection to the maintenance of the patent. This issue is important since in appeal stage fresh grounds of oppositions may in principle not be introduced, unless the patent proprietor agrees.
The ground of opposition under Art. 100(b) - lack of sufficient disclosure - is quite frequently brought up in EP Opposition procedures, although it is often misconstrued. Many times this ground is forwarded because an opponent is of the opinion that the claims do not allow the skilled person to practice the invention. However, for an attack based on this ground it is not sufficient to only consider the claims. Rather, this ground can only lead to a revocation of the patent, if the patent as a whole does not sufficiently disclose the invention. A typical case where Art. 100(b) can lead to the revocation of a patent is the situation where the claims recite a parameter which is not properly defined in the specification.
Art. 100(c) -added subject matter- provides fora very delicate ground of opposition, both for the EPO examiner and the person who has prosecuted the case. While simple additions can most often easily be removed and thus a total revocation can be avoided, there is also the less favorable situation of the “inescapable trap“. This situation arises if the main claim contains non-disclosed matter, i.e. added matter, which limits the protective scope of the patent. Such matter cannot remain in the claim in view of Art. 100(c), but it also cannot be removed from the claim, as this would lead to a broadening of the protective scope. Such a broadening of the protective scope after grant is not allowable under Article 123(3) EPC. This clearly leads to a no-win situation for the patentee and it will invariably lead to the revocation of the patent.
4. Written Proceedings
Once the opposition has been filed, upon examination of the formalities it will be brought to the attention of an Opposition Division. The Opposition Division is composed of three EPO examiners, at least two of whom have not taken part in the Grant Proceedings of the patent to which the Opposition relates (Art. 19(2)). A first member of the Opposition Division, who is usually the most experienced and never the examiner who was in charge of the case prior to grant, will be the chairman. As the examiner who examined the case is, of course, most acquainted with the case, he or she is usually entrusted with the Opposition case and the preparations prior to making the decision. This second member of the Opposition Division is usually called the rapporteur. A third member will usually be the minute’s writer in the event of Oral Proceedings.
According to Art. 101(1), if the opposition is admissible, the Opposition Division shall examine, in accordance with the Implementing Regulations, whether at least one ground for opposition under Art. 100 prejudices the maintenance of the European patent. During this examination, the Opposition Division shall invite the parties, as often as necessary, to file observations on communications from another party or issued by itself.
In accordance with Rule 79(1) the Opposition Division shall communicate the notice of opposition to the proprietor of the patent and shall give him the opportunity to file his observations and to amend, where appropriate, the description, claims and drawings within a period to be specified. In return, patentee will usually file a response outlining his defense. If several notices of opposition have been filed the patent proprietor may respond to several opponents by one statement only, or he may also file separate statements responding to each of the opponents.
If several notices of opposition have been filed, the Opposition Division shall communicate them to the other opponents in accordance with Rule 79(2).
Following Rule 79(3) the Opposition Division shall communicate any observations and amendments filed by the proprietor of the patent to the other parties, and shall invite them, if it considers this expedient, to reply within a period to be specified.
Accordingly, it is the opponent‘s turn to once again respond, and so forth. Usually two to three written statements are forwarded by each side. While the opponent may certainly react to patentee’s response, in principle he may not take a “second shot“ in the sense that he introduces new material and even new grounds of opposition, unless this is motivated by the patentee‘s response.
EPO case law in an attempt to expedite Opposition Proceedings and to avoid piecemeal argumentation for tactical reasons, has imposed rather strict procedural limitations in this respect. That is, in principle, the grounds of Opposition forwarded and substantiated in the initial notice of Opposition and the evidence contained therein, set the legal and factual framework for the entire Opposition Proceedings (G 9/91 and G 10/91).
Additional grounds (n.b. EPO’s interpretation of “grounds“ above) need not be considered by the Opposition Division. However, it is at the discretion of the Opposition Division to do so under Article 114 (1) and Rule 81 EPC. According to the Enlarged Board of Appeal, however, exercising this discretion must be limited to exceptional circumstances, where there are prima facie strong reasons that speak in favor of the relevance of the respective grounds of opposition and against the maintenance of the patent in its entirety (G 10/91). Similarly, claims that were not attacked in the original notice of Opposition are not the subject of Opposition Proceedings. While dependent claims may still be examined by the Opposition Division in the case where their patentability is prima facie in question on the basis of the available material, independent claims may not.
Prior art that was not introduced within the nine months Opposition period may also be disregarded by the EPO for belated filing(T 1002/92). Accepting documents that were filed later is, again, purely at the discretion of the Opposition Division, and this discretion has to be exercised on the basis of a prima facie analysis of the relevance of the late filed documents.
These strict procedural rules show that opposition proceedings are contentious Revocation Proceedings rather than a continuation of the pre-grant examination procedure. The principle that the EPO examines the facts of its own motion as laid down in Article 114(1) EPC is thereby basically sacrificed for procedural reasons.
In Opposition Proceedings, the EPO, therefore, basically no longer acts as a patent office but, rather, as a court. In fact, G 9/91 rendered it clear that nowadays the European Opposition is to be considered as a truly contentious proceeding, wherein the conflicting interests of the patentee and the opponent(s) have to be decided upon. It is therefore also not surprising that the EPO’s practice of Rule 84(2) EPC, which prescribes that the Opposition Proceedings may be continued even if opponent withdraws, is such that the withdrawal of the opponent (or all opponents in the case of more than one opponent) marks the end of the proceedings unless there is a clear cut lack of novelty.
The written proceedings may extend over a period of 2 to 3 years. The Opposition Division may then issue a decision on the basis of the written statements only. However, in the vast majority of cases, the decision will be made following an Oral Hearing.
5. Oral Proceedings
Intheirbasiclayout, the European Opposition Proceedingsarewrittenproceedings. Oral Proceedings are not compulsory. However, Opposition Proceedings rarely follow this basic layout. It is a rather common practice for both the opponent and the patentee to routinely request Oral Proceedings under Article 116 (1) EPC. Once such a request has been filed, the EPO has no discretion; it must summon for Oral Proceedings.
The summons to Oral Proceedings must be sent by the EPO at least two months in advance of the Oral Proceedings (Rule 115). Nowadays, however, such summons is usually issued many months earlier.
There is, however, also a legal reason why the EPO issues the summons to Oral Proceedings rather timely. This reason is Rule 116 EPC, which yet again shows the EPO‘s preference of procedural efficiency over the aim of ultimate quality of examination:
(1) When issuing the summons, the European Patent Office shall draw attention to the points which in its opinion need to be discussed for the purposes of the decision to be taken. At the same time a final date for making written submissions in preparation for the Oral Proceedings shall be fixed. Rule 132 shall not apply. New facts and evidence presented after that date need not be considered, unless admitted on the grounds that the subject of the proceedings has changed.
(2) If the applicant or patent proprietor has been notified of the grounds prejudicing the grant or maintenance of the patent, he may be invited to submit, by the date specified in paragraph 1, second sentence, documents which meet the requirements of the Convention. Paragraph 1, third and fourth sentences, shall apply mutatis mutandis.
Rule 116 EPC, of course, also aims at setting the framework for fair play amongst the parties by forcing them to present their case early and, thus, allowing for the sufficient preparation on the part of the EPO and the other parties before the Oral Proceedings.
A further issue is the language of the proceedings. Most of all oppositions are lodged in the German or English language. The majority of opponents thus plead and speak in these languages though the language of the proceedings (the language used by the Opposition Division) may be different. The language of the proceedings is the language in which the attacked patent was issued (predominantly English). For the Oral Hearing (only), the EPO will provide a translation service at its own expense if this is requested by the parties or the Opposition Division. There will be simultaneous translation of what is said in the proceedings. These translations can be followed by headphones.
The Oral Hearing is open to the public. The actual Oral Proceedings is much like a court case. Usually the Chairman gives a short introduction into the case and then the opponent is given the opportunity to present his case. The patentee may, of course, reply, and so forth. It is thereby not rare that members of the Opposition Division address specific questions to the parties.
The burden of proof is firstly with the opponent. As the opponent is the party that initiated the Opposition Proceedings it is up to him to show that his allegations are probable. If he complies with this requirement, for example by forwarding compelling experimental evidence, the burden of proof may shift to the patent proprietor.
Once the arguments are exchanged, the Opposition Division will interrupt the Oral Proceedings and retreat for internal discussion. In most cases, the Chairman upon reopening the proceedings will then issue a decision or, less often, continue with the discussion. It is also not unusual that in Oral Proceedings the case is dealt with in a step-by-step fashion, whereby formalities and the individual grounds of opposition, i.e. in most cases novelty and inventive step, are dealt with and decided upon individually, one after the other.
As a basis of its decision, the Opposition Division must adhere to the text, i.e. the requests forwarded by the patentee. The Opposition Division can only decide whether or not these requests are in conformity with the EPC (Article 113(2) EPC). Accordingly, even if the Opposition Division is of the opinion that a slight change could render the claims proposed by patentee patentable, it may not affect this change themselves. If patentee is not willing to affect this change or fails to notice respective hints given by the Opposition Division, the Opposition Division has no choice but to revoke the patent.
6. Amendments
In Opposition Proceedings, the patent proprietor is entitled to amend his patent. These amendments are subject to Article 123(2) and (3) EPC, and impose the following restrictions:
- the European patent may not be amended in such a way that it contains subject matter which extends beyond the content of the application as filed, and
- the claims of the European patent may not be amended during Opposition Proceedings so that they extend the scope of protection.
If the patent proprietor wishes an amendment to the claims, he may use the entire original application text as a basis. He is not limited to what is already contained in the claims. He is free to insert features from the description into the claims, provided that their scope is not extended thereby.
Rule 80 EPC concerns amendments to a patent granted by the EPO and under opposition. It is expressed in permissive language to allow the patent proprietor to amend whenever “the amendments are occasioned by grounds for opposition specified in EPC Article 100”. Decisions of the EPO Boards of Appeal in the past have restricted the scope for amendments during opposition to amendments necessary to overcome the particular attacks from the opponent. The opposition process is not to be regarded as an opportunity for a patent proprietor to make a general review and revision of the text of his patent, unrelated to the issues raised by the opponent. The mere addition of new claims to the granted claims is inadmissible since such an amendment cannot be seen as meeting the grounds for opposition. However, Rule 80 EPC gives the proprietor permission to make any amendment which can be justified as a response to a “theoretical” ground of opposition “even if the respective ground has not been invoked by the opponent”. Thus, if the proprietor is aware of potential, but not yet actual grounds for opposition to his patent, he should be able to rely on this rule for permission to make remedial amendments to his patent, even though the actual opponent has not raised the same attack. For example, in opposition proceedings admissibly opened on the grounds of patentability, the patent proprietor may submit amendments to remove added subject-matter.
7. Intervention of the Assumed Infringer
Art. 105 (1) enables any third party to intervene in opposition proceedings after the opposition period has expired, if the third party proves that proceedings for infringement of the same patent have been instituted against him, or following a request of the proprietor of the patent to cease alleged infringement, the third party has instituted proceedings for a ruling that he is not infringing the patent. According to Art. 105(2) the EPO will treat an admissible intervention as an opposition. In accordance with Rule 89(1) EPC, the notice of intervention should be filed within three months of the date on which proceedings referred to in Art. 105 EPC are instituted. The filing of the notice of intervention requires the payment of the opposition fee.
Intervention is permissible as long as opposition or appeal proceedings are pending. A third party can become a party to the proceedings during the period for filing an appeal only if a party to the proceedings in which the decision was given files an appeal pursuant to Art. 107; otherwise the decision of the Opposition Division will become final on expiry of the appeal period (G 4/91 and G 1/94).
A properly filed and admissible intervention is treated as an opposition, which may be based on any ground of opposition under Art. 100 (G 1/94). This means that, when intervening at any stage of first-instance proceedings, the intervener enjoys essentially the same rights as any other party to the proceedings. If the intervener introduces new facts and evidence which appear to be crucial, the proceedings may need to be prolonged to enable them to be adequately considered.
8. Conclusion of the Opposition Procedure
At the end of the opposition procedure the Opposition Division may reject the opposition, revoke the patent or maintain the patent in an amended form (Art. 101(2)(3)).
The decision of the Opposition Division may be appealed by the party or parties adversely affected by it by filing an appeal with the EPO within two months after the notification of the respective decision. The appeal is forwarded to the Appeal Board that issues a final decision.
The effect of decisions issued by the Opposition Division (with no appeal filed) and those issued by the Appeal Board is set forth in Art. 68 that defines that the European patent application and the resulting patent shall be deemed not to have had, from the outset, the effects specified in Articles 64 and 67, to the extent that the patent has been revoked or limited in opposition limitation or revocation proceedings.
Beyond this, however, the decision has no effect. That is, in particular, it does not prevent the unsuccessful opponent from filing national revocation actions on exactly the same grounds and with exactly the same prior art as he did before the EPO. Decisions issued in EPO Opposition Proceedings do not have a binding effect on the parties; the matter is not res judicata for national revocation courts.
Accordingly, the unsuccessful opponent has a second, though laborious and expensive chance to reach his goal nationally.
For the unsuccessful patentee, however, there is no second chance. The patent is gone and cannot be revived nationally.
II. Appeals
- Introduction
- The Boards of Appeal
- Initiating an Appeal
- Subject and Scope of the Appeal
- Parties to the Proceedings
- How Appeal Proceedings are Conducted
- Time and Cost of Proceedings
- Binding Effect of Previous Decisions
- The Enlarged Board of Appeal
1. Introduction
In proceedings before the EPO, a party has the right to appeal against a first instance decision which has gone against him.
Broadly speaking, there are two types of appeal cases which are common.
The first is referred to as ex parte appeals; these are appeal cases solely between a party to proceedings at the EPO and the EPO itself. A typical example of such an appeal would be a case where a patent application has been refused by an examining division, and the applicant considers that the reasoning given is not correct or fair.
The second type is referred to as inter partes appeals; these are appeals in cases where there are two or more adversarial parties, typically this would relate to an EPO opposition case.
2. The Boards of Appeal
From an organizational perspective, the boards of appeal are separate from other departments of the European patent office. According to the EPO organizational structure, the EPO is divided into five directorates-general (DG), where DG 1 is responsible for e.g. first instance examination and opposition proceedings and DG 3 is responsible for appeals. The boards of appeal are based in Munich, where all appeal hearings take place.
The work done by the boards of appeal is done by the so-called members of the boards of appeal. These are EPO employees who have a time-limited contract of usually five years (which can be extended), and who have either a technical degree or a legal degree. The members of the boards of appeal are typically chosen amongst the best and most experienced examiners, and external recruitment also takes place. It is generally considered that the members of the boards of appeal are effectively judges of European patent law and they operate with significant independence from the EPO. They only work on appeal cases.
The boards of appeal are divided up into a number of specific boards. There are at present 28 technical boards of appeal, each of which is populated by a number of technical members and a number of legal members. The technical members have a relevant science degree, and the legal members have a degree in law. There is also a legal board of appeal and an enlarged board of appeal, populated by certain members of the boards of appeal who also sit on the technical boards of appeal. We will return to the function of the enlarged board of appeal later. When an appeal is filed, it is assigned to a board of appeal, usually to one of the technical boards. The assignment to a particular technical board is done on the basis of the IPC class of the application or the patent to which the appeal relates; this ensures that the appeal is handled by a board with the appropriate technical knowledge. There is a pre-existing business distribution scheme which is published by the EPO each year which defines which boards are responsible for which IPC classes.
If an appeal relates exclusively to a legal issue, it will be referred to the legal board of appeal rather than to a technical board of appeal.
For a given case, the responsible technical board of appeal will assign a panel of three members to hear and decide on the case. The panel consists of three members (with the option for expansion if needed); at least two technically- qualified members and one legally qualified member are present. In contrast, a legal board of appeal would consist of three legally-qualified members.
Within the three-man panel, one member is assigned as the chairman. It is the chairman’s responsibility to conduct the proceedings fairly. Typically, each board only has one chairman so that he will sit in on every case assigned to this board. In addition to the chairman, there will be a first member assigned; he will typically be a technically qualified member, and he will be principally responsible for studying the written arguments and evidence of the case, briefing his two colleagues in advance of the hearing and for writing the reasons for the decision. Each board will have several technically qualified members, so that they rotate and each take part in fewer cases per year than the chairman. The third member of the panel is typically the legal member, whose task it is to focus on legal aspects of the case. As can be expected, the extent to which complex legal issues come up varies much from case to case, and of course the technical members are very experienced in substantive patent law. Therefore, the legal member is not always so heavily involved in the decision-making process. It is a legal requirement that there is a legally-qualified member present in each appeal hearing.
3. Initiating an Appeal
An appeal can be filed against any final decision of an EPO first instance by any party who is adversely affected by that decision. A party is adversely affected if his main request was not granted to him by the EPO. Thus, in EPO opposition proceedings a patentee can appeal if his patent was revoked or if it was maintained on the basis of an auxiliary request. Similarly, an opponent can appeal if he had requested complete revocation and the EPO decided to maintain the patent in any form. It can therefore happen that an opposition case has an intermediate outcome which is appealed both by the opponent and by the patentee. If an appeal is filed from an opposition case and there is only one appellant, then the non-appealing party to the opposition is still a party in the appeal proceedings where he is referred to as a respondent.
There are two essential steps that are required to initiate an appeal, namely the filing of (i) a notice of appeal and (ii) the grounds of appeal.
3.1 The Notice of Appeal
The notice of appeal consists of a statement that an adversely affected party wishes to appeal against a certain first instance decision. It must be accompanied by the appeal fee. The notice of appeal must be filed within two months of notification of the written decision which is being appealed. This deadline is not extensible.
3.2 The Grounds of Appeal
The grounds of appeal consist of a reasoned statement explaining why the decision under appeal is wrong and why it should be overturned. According to the rules of procedure of the boards of appeal, an appellant must set out his full case in his grounds of appeal. The deadline for filing the grounds of appeal is four months after notification of the written decision which is being appealed. This deadline is also not extensible.
4. Subject and Scope of the Appeal
In some EPC member states, e.g. the UK, the purpose of an appeal is to provide a review of the legal correctness of the first instance decision. Therefore, in British appeal proceedings, the appellant is essentially restricted to presenting arguments relating to the application of the law by the first instance Judge. However, arguments which relate to factual matters would not be accepted and generally new evidence cannot be filed. Other EPC member states, e.g. Germany, take a different approach and effectively allow an appellant to re-run his case so that it is possible to also argue that the first instance decision is wrong due to a factual error, and the filing of new evidence might also be possible.
The European Patent Convention itself does not expressly define which types of arguments an appellant should be allowed to rely on and as such there is a somewhat diverging practice between different EPO appeal boards. Generally speaking, the EPO boards of appeal are relatively generous and flexible when it comes to accepting new material in appeal proceedings. Thus, it is often possible for an applicant or a patentee to file new amended claims during an appeal, or in general for parties to file new arguments or new evidence. It is always possible in an appeal to challenge the correctness of factual findings by the first instance.
This characteristic of EPO appeal proceedings, coupled with the relatively low cost results in a rather large number of appeals is being filed each year.
5. Parties to the Proceedings
As mentioned above, the parties to an EPO appeal may include one or more appellants and one or more respondents. As will be discussed below, whether a party is an appellant or a respondent can make a difference.
By definition, an appellant is any party who files an admissible appeal in time.
A respondent is any party who is not an appellant but was a party to the proceedings resulting in the decision under appeal.
5.1 Relevance of Party Status – Prohibition of Reformatio in Peius
Whether a party is classified as an appellant or a respondent can have an impact on the legal flexibility of that party during appeal proceedings. This is because of a well-established legal principle referred to as the prohibition of reformatioinpeius. This principle excludes the possibility that the sole appellant in EPO proceedings can be left worse off as a result of the appeal. Put differently, it means that if an appellant is the only appellant, then he is guaranteed an overall outcome which is at least as good as the outcome of the first instance decision.
As will be appreciated, this principle is only relevant in interpartes proceedings, and where the outcome of the first instance was an intermediate result maintaining the patent in amended form on the basis of an auxiliary request.
In this situation, if both parties appeal, the outcome of the appeal is entirely open and could be anywhere in the range from maintenance of the patent as granted to complete revocation.
However, if only the opponent had appealed, then the opponent would be guaranteed that the patent could not be maintained with any claims which are broader than those which were left at the end of the first instance proceedings. Conversely, if the patentee was the sole appellant, patentee would be guaranteed to keep those claims and so the sole subject of the appeal would be to decide whether broader claims could be allowed.
In order to avoid this restriction, in opposition cases where the result is an intermediate one so that neither party wins, it is typical for both parties to appeal.
5.2 Third Party Observations
Anyone who is not party to the proceedings can still contribute by making third party observations. These observations can be submitted anonymously, and can contain arguments and evidence relating to the ongoing appeal. Those can then be taken over by the parties to the appeal, or by the appeal board itself.
5.3 Intervention of the Assumed Infringer
As discussed in the preceding chapter on opposition proceedings, a party who has been sued for infringement in an EPC member state may join an ongoing opposition against that patent, even if he did not oppose in time. It is also possible for the alleged infringer to join during an ongoing appeal relating to an opposition case.
6. How Appeal Proceedings are Conducted
6.1 Written and Oral Proceedings
In both ex parte and inter partes appeals, there will typically be an initial phase of written argument exchange (written proceedings) followed by a hearing (oral proceedings). The decision will typically be announced at the end of the hearing, and written reasoning for the decision will follow later. In more detail:
6.2 Ex Parte Appeals
In ex parte appeals, there is only one party to the appeal. Typically, the filing of the grounds of appeal will be followed by a relatively long period of inactivity until the board of appeal issues a summons to oral proceedings. The hearing date is decided by the board of appeal and the appellant is informed of this in advance in writing. The board of appeal has the option of providing intermediate written communications to the appellant, and they may also provide a preliminary opinion on the case in advance of the hearing. Such communications serve to highlight any issues which the board of appeal primarily considers to be key points on which the appellant so far has not managed to ensure compliance with the EPC.
Oral proceedings will only take place if the appellant has requested a hearing (which is a routine measure). Typically, a hearing request will be conditional meaning that the appellant makes it clear that no hearing is wanted or needed if the EPO appeal board is minded to reverse the decision under appeal. Then a written decision in the appellant’s favour can be issued without the need for a hearing to take place.
If a hearing does take place, this will not be open to the public. The hearing will take place in a closed hearing room; it is usually be set for one day, starting first thing in the morning and concluding once the discussion is complete. The hearing will be attended by the members of the boards of appeal responsible for deciding the case as well as the applicant’s European patent attorney and optionally some representatives from the applicant (e.g. technical experts, the inventor or an in-house patent attorney). Usually an ex parte appeal hearing might take 1-5 hours. At the end of the hearing, a decision will almost always be announced. Possible outcomes include the dismissal of the appeal (in which case the examining division’s refusal of the appeal is finally confirmed), a decision to allow the appeal and overturn the contested decision (in which case a patent would be granted) or the case could be partially decided on some issues only and then remitted to the first instance to continue examination.
Generally speaking, it is often difficult to persuade an appeal board that an EPO examining division was overly tough, and so quite a lot of ex parte appeals are not successful. Of course, the chance of success is strongly dependent on the case in question. So if the examiner did make a mistake, this can be corrected by filing an appeal, and if the applicant is able to cure deficiencies in the application by bringing new arguments, evidence or amendments this may also lead to success.
A written decision is issued at a later point in time, typically 1 to 2 months after the hearing. This will contain the detailed reasons why the board of appeal decided the way it did.
The boards of appeal are the highest EPO instance, which means that no further appeal is possible and that the decisions issued are final.
6.3 Inter Partes Appeals
In inter partes appeals, the proceedings are somewhat more complicated. As mentioned above, the filing of the notice of appeal and grounds of appeal initiates appeal proceedings. Once the EPO receives the grounds of appeal, this will be forwarded to the other parties. The other parties will be set a deadline (usually four months) for the filing of a response to the grounds of appeal. This deadline may theoretically be extended, although the practice varies from board to board as to whether or not such discretionary extensions are given.
The grounds of appeal or the response to the grounds of appeal is a very important brief, because the rules of procedure of the boards of appeal specify that the grounds of appeal and/or the response to the grounds of appeal shall set out a party’s full case. In other words, this means that a party is not free to amend his case by adding new claim amendments, arguments or evidence at a later point in time. If a party nevertheless does wish to amend his case later, any new amendments, arguments or evidence will be admitted only on a discretionary basis, the decision on admission being dependent on the surrounding circumstances (e.g. relevance of the new material, the increase in complexity it causes, procedural fairness, timing of the filing and reasons for the lateness).
Once the EPO receives the response to the grounds of appeal, this will also be forwarded to all the other parties to the proceedings. Typically, no deadline will be set for further written submissions although the other parties are free to make further written submissions if they want to. Eventually, time will come for oral proceedings to be held. As in ex parte cases, oral proceedings are held only at the request of the parties. However, in opposition appeal cases the patentee will typically request a hearing if the patent cannot be maintained as granted (or at least on the basis of the main request), while the opponents will request a hearing if the patent cannot be completely revoked. In this scenario, a hearing is inevitable.
When the appeal board is ready for the hearing to be held, it will notify the parties by issuing a summons to oral proceedings at least two months before the intended hearing date. The board of appeal will have the option to include e.g. with the summons a preliminary opinion which sets out the board’s preliminary view of the case and highlights key issues for discussion during the hearing. Whether or not such a preliminary opinion is provided is discretionary and here practice varies significantly from board to board and member to member. It is however not at all unusual for the board to decide not to issue any communication to the parties other than the summons which includes only practical details about where and when the hearing will take place.
An inter partes hearing at the EPO is public meaning that anyone can attend. Naturally, the members of the boards of appeal responsible for deciding the case will be present. Each party to the proceedings will also have been invited, and will usually be represented by their European patent attorney, optionally accompanied by one or more representatives from their client company (e.g. technical experts, in-house patent attorneys etc.).
The chairman of the board of appeal will be responsible for conducting the proceedings and it will be his decision in which order the various topics on the agenda are to be discussed. It is also the chairman who decides when the parties are allowed to address the board. Typically, the debate is structured by discussing one topic at a time. Usually, the chairman will focus on the decision under appeal and will require the appellant to explain why he believes that this decision should be overturned. The other parties to the proceedings will of course also have an opportunity to present their point of view and to explain why the decision should stand, and they may also present other arguments to support their overall case.
Generally speaking, the case presentation is done by the parties’ respective European Patent Attorneys. Although there are provisions for witnesses to be heard and for expert testimonies to be given, these are not often used.
Once a certain topic has been discussed and all parties have had a reasonable chance to present their views on that topic, the board will typically choose to deliberate on that topic. For the deliberation, everyone except the members of the board will leave the room and the board will reach a decision on the points which were discussed. When the hearing resumes, the board will usually announce ist decision on that point to the parties and the chairman will then decide how the debate is going to continue.
Broadly speaking, the board will usually focus on the main request and auxiliary requests of the patentee, and will assess these one at a time starting with the main request (i.e. the highest ranking request, which will usually have the broadest claims). The examination of a particular request will come to an end either when one argument against the validity of those claims is found by the board to be justified, or when all arguments against the validity of the claims have been discussed and found to be unfounded. If the claims are found not to be valid, the discussion will move on to the next request on file. If the claims under discussion are found to be valid, then the patent will be maintained on the basis of those amended claims.
It is generally difficult for patentee to amend his claims during the oral proceedings (unlike in the first instance, where this is not so uncommon). Therefore, in EPO appeal proceedings it is very important for patentee to consider his fall-back positions at an early stage and file appropriate auxiliary requests.
Inter partes appeal hearings vary in length, this depending very greatly on the complexity of the case and the number of parties involved. Typically, if there are more than three parties involved or if the case is very complex, the hearing will be set for two consecutive days. A normal opposition appeal will only be set for one hearing day.
At the end of the oral proceedings, the board of appeal will typically announce a final decision. Broadly speaking, the board of appeal can decide to revoke the patent, to maintain it in amended form or to maintain the patent as granted. They also have the option to only decide one aspect of the case and then to remit the case to the first instance for further examination. This means that EPO opposition proceedings would resume. The willingness to remit cases is rather variable from board to board; a typical scenario where a case might well be remitted is where the first instance decision only decides on certain EPC requirements, and where the appeal board subsequently finds that decision to be wrong. For example, if a first instance decision says that all requests lack novelty but makes no comment on inventive step, then the case is very likely to be remitted if the appellant can convince the board that the claims are novel. This is because there is a general principle that a party to proceedings before the EPO must have the possibility to have his case considered by two instances. However, the appeal boards do also understand that EPO opposition and appeal proceedings already take a very long time and that a decision to remit the case will significantly prolong these proceedings. In an average case, a remittal might cause a delay of 3-4 years in proceedings that are already lengthy.
While it is not so meaningful to provide statistics on the outcome of appeal proceedings, it can generally be said that the result at the end of the appeal is often different than that at the end of the opposition. Sometimes this is because the appeal board thinks that the first instance decision was not correct, and sometimes this is because the case has changed (in that new evidence, arguments or amendments are presented). Also for this reason, the filing of an appeal at the end of an opposition case is a relatively routine measure for many users of the EPO system.
A written decision is issued at a later point in time, typically 1 to 2 months after the hearing. This will contain the detailed reasons why the board of appeal decided the way it did.
All decisions of the boards of appeal are published online and each is assigned a case number which has the format T XXX/YY for the technical board of appeal decisions and J XXX/YY for the decisions of the legal board of appeal. YY represents the year in which the appeal was filed and the integers XXX represent a serial number. The most important decisions are published in the EPO Official Journal, and the EPO also publishes a case law book which summarizes the most important decisions which have an impact on current EPO practice.
7. Time and Cost of Proceedings
7.1 Time to Completion
An EPO appeal typically takes 2-4 years to complete. Appeals are usually handled by the responsible board based on the order in which the appeals were filed. The duration of an appeal is therefore strongly dependent on the workload of the board to which the appeal is assigned, and so it can vary from one technical field to another.
7.2 Acceleration
Acceleration of appeal proceedings can be requested. While the EPO will consider any request, it is only if there is ongoing infringement litigation in an EPC member state that there is a high likelihood that the board of appeal will put that case at the front of its queue of cases. Acceleration can have quite an impact on speed, and will allow the appeal to be resolved much more quickly. If acceleration is granted from the start of the appeal, the case might be resolved within a year or so. If acceleration is granted while the appeal is ongoing, the appeal board will typically immediately schedule a hearing for the first available date. A request for acceleration can be filed by one of the parties or e.g. by a judge in a national court where the national litigation may be stayed to await the outcome of the EPO case.
7.3 Costs
The costs for an EPO appeal are the official fee (€ 1880 in March 2017) plus the costs for the European Patent Attorney handling the case.
Legal costs for an EPO appeal are usually strictly handled by requiring each party to pay their own costs. There is typically no possibility for the winner to collect costs from the loser, contrary to the situation in many national courts in EPC members states, e.g. Germany and the UK. Although there is a provision for the award of costs in EPO proceedings, this is used extremely rarely.
Overall, costs of an EPO appeal are moderate or even low when compared to e.g. the costs of national patent litigation in key EPC member states such as Germany and the UK.
8. Binding Effect of Previous Decisions
Common law jurisdictions such as the UK operate with a system of binding precedent. This means that, when a court is deciding on a given case, it must take into account and follow earlier decisions on the same legal point from the same court or from higher courts.
However, the EPO does not operate on this basis. Thus, an individual board of appeal has a high degree of freedom as regards how it decides a case before it.
Specifically, an earlier decision of a technical board of appeal or a legal board of appeal is not binding on an appeal board for which reason a deviation from certain principles previously established is possible.
In practice, it is fair to say that the EPO boards of appeal do aim at the establishment of mainstream case law which is the result of continuous refinement of principles and practices established through previous decisions. Therefore, quoting from earlier decisions can often be persuasive.
As will be discussed in the section below, the enlarged board of appeal also issues decisions and opinions. These decisions are also not strictly binding on boards of appeal in other cases, although the boards of appeal have less flexibility to decide the case in a manner which is in direct contradiction with a key principle from an earlier decision or opinion of the enlarged board of appeal. In such a situation, the board of appeal would be required to submit a further referral to the enlarged board of appeal for reconsideration of the legal issue at hand.
9. The Enlarged Board of Appeal
The enlarged board of appeal is made up by members of the boards of appeal. Typically, the members of the enlarged board are the most senior members of the boards of appeal and many are chairmen of their individual technical boards.
The enlarged board of appeal is not a further appeal instance in addition to e.g. the technical boards of appeal. The enlarged board instead has two other specific roles, namely to address specific referrals on points of law and to decide on so- called petitions for review.
9.1 Decisions of the Enlarged Board of Appeal
From time to time, e.g. in proceedings before the boards of appeal, a legal issue will come up which the responsible board of appeal considers difficult to resolve on its own. This difficulty might result from the fact that the same issue has been resolved differently in various earlier decisions, or because it is a particularly complex legal issue. In those cases, the board of appeal may choose to refer a question of law to the enlarged board of appeal.
When such a referral is made, appeal proceedings are temporarily interrupted and proceedings before the enlarged board initiated. The proceedings before the enlarged board will be aimed at answering the legal question which has been posed by the referring board, and in deciding how to answer the questions the enlarged boardwilltake intoaccount submissions by theparties totheproceedings as well as submissions by other interested parties such as e.g. non-governmental organizations, bar associations or industry associations. These proceedings could last several years and will result in a written opinion or decision providing the answer to the questions that have been posed. The case will then go back to the referring appeal board which will complete the case applying the answers that have been provided.
Decisions of the enlarged board of appeal are also published and are assigned a case number with the format G XXX/YY, where YY is the year of the referral and XXX is a serial number.
9.2 Petitions for Review
In addition to providing decisions on interesting points of law, the enlarged board of appeal is also responsible for reviewing and deciding on so-called petitions for review. It is mentioned above that a decision of the board of appeal is a final decision and that there is no further EPO instance. The petition for review process provides for the possibility that a case can be continued even after a final decision by a board of appeal; however, this is possible only in very rare circumstances. Specifically, this system is meanttoprovide a safeguardagainst procedural mistakes or gross unfairness in connection with a completed appeal, and effectively allows for a retrial if a party can prove that such a serious mistake took place. As will be evident, this provision of the law is meant to provide a possibility for a retrial only in exceptional circumstances, for which reason the number of successful petitions for review is predictably small.
III. Central Limitation and Revocation Procedure
- Overview
- Why limit or revoke a granted patent?
- What can and cannot be limited by amendment?
- When can amendments be made?
- How is a request for central limitation or revocation made?
- How does the EPO examine a request for central limitation or revocation?
- Obtaining Different Claims for Separate Contracting States
- Successful Request
- Unsuccessful Request
1. Overview
Central limitation and central revocation procedures were introduced into European patent law in December 2007 as part of the EPC2000 package. These procedures respectively enable the proprietor of a European patent to request limitation of the patent claims or complete revocation of the patent. Operation of these procedures has been without legal controversy evidenced by the fact that there have been no decisions from the Technical Boards of Appeal on their operation in the intervening several years.
Central limitation provides a convenient and cost-effective way for a patent proprietor to restrict the scope of their patent after it has been granted. In the early years of the EPC, patent proprietors seeking to limit their patent centrally at the EPO could self-oppose, provided that this was done within 9 months of grant. However, self-opposition was outlawed by the EPO Enlarged Board of Appeal Decision (G 9/93). The national patent offices of some EPC contracting states (notably those of the UK, Germany, the Netherlands, France, Italy and Spain) permit post-grant claim limitation, but a claim limitation conducted by a national patent office is only effective in that contracting state. Following this path it is both expensive and inconvenient as it requires coordinating parallel limitation procedures with each national patent office. The introduction of a central limitation procedure was therefore a welcome addition to the options available to patent proprietors.
The EPO central limitation and revocation procedures are relatively speedy. If a request for central limitation is clearly allowable, the time taken for the entire procedure is approximately three to six months. If, however, the limitation gives rise to objections, then the procedure can take a year or more. The procedure for central revocation is simpler than the central limitation procedure, and typically takes from two to four months.
2. Why limit or revoke a granted patent?
The most common reason for limiting the scope of a granted patent is to ensure that its claims are novel and inventive over prior art that was not considered by the EPO Examiner during examination. National prior rights (national applications that are pending but unpublished at the effective priority date of a patent) are one example of this type of prior art, as EPO Examiners rarely cite such prior art during EPO examination proceedings. This is because national prior rights do not constitute prior art under the European Patent Convention. National prior rights do, however, belong to the state of the art in their national jurisdiction, hence it is necessary to ensure that the claims in each EPC contracting state are valid over any local national prior rights. The threat of national prior rights is set to become more significant when the Unitary Patent (European patent with unitary effect) enters into force, as it is possible for a single national prior right to invalidate an entire Unitary Patent. The central limitation procedure may be used in the future to prevent the total revocation of a Unitary Patent on the basis of a national prior right.
A patent proprietor may also amend the granted claims in order to establish novelty and an inventive step over the more usual type of pre-published prior art, particularly documents which first come to the proprietor’s attention post-grant.
Alternatively, a patent proprietor might choose to limit the claims as granted so that they are more closely tailored to the product or process of an infringing party. Although this sacrifices claim scope, it decreases the likelihood that the infringing party will be able to respond to an infringement action by citing invalidating prior art when counterclaiming for revocation during litigation. Limiting the claims in this way may therefore be a useful strategy when preparing to sue for patent infringement.
The reasons for requesting central revocation at the EPO are few, since it is possible to passively abandon a patent across Europe by neglecting to pay renewal fees if, for example, the patent is either no longer required or is found to be incurably invalid. However, central revocation at the EPO affords a quicker and more decisive option than allowing a patent to become abandoned through inaction. Moreover, central revocation via the EPO has retroactive effect, meaning that the patent is deemed never to have existed. Central revocation might therefore be a potential bargaining point when negotiating with third parties.
3. What can and cannot be limited by amendment?
As central limitation is concerned with the protective scope of a patent, it is primarily concerned with the claims. The description can only be amended if this is necessary to ensure that it is consistent with claims that are amended as a result of the central limitation procedure.
Not all claim amendments are acceptable. First and foremost, the claims resulting from an amendment must comply with all of the usual formal requirements of the European Patent Convention such as clarity, sufficiency and prohibition against adding subject-matter. It is also necessary that the claim amendments must effect a limitation. This means that it must be impossible to imagine an embodiment which falls within the scope of the limited claims but which did not fall within the scope of the claims as granted. Claim amendments that simply ‘tidy up’ or attempt to clarify the claims will be rejected; so too will amendments that merely introduce extra dependent claims. This however does not preclude amendments being made solely to limit the scope of dependent claims (Guidelines D-X 4.3).
4. When can amendments be made?
Central limitation can be requested at any time after the grant of a European patent, with one important exception. Central limitation cannot be requested if EPO opposition proceedings are pending (Article 105a(2) EPC). If an opposition is filed against a patent that is already the subject of pending limitation proceedings, the limitation proceedings are terminated and the limitation fee is refunded (Rule 93(2) EPC).
A valid request for central revocation cannot be filed if opposition proceedings are pending (Article 105a(2) EPC). However, a proprietor is able to terminate the opposition proceedings in a relatively quick fashion by filing a statement at the EPO disapproving the granted text without filing a replacement text in its place. Filing a statement of this kind leads to the rapid revocation of the opposed patent by the EPO.
Central limitation proceedings do not take precedence over national proceedings, such as revocation proceedings, that are taking place in contracting states where the patent is in force. However, as central limitation has retroactive effect, many jurisdictions will stay their national proceedings pending the outcome of the central limitation procedure.
5. How is a request for central limitation or revocation made?
Central limitation and central revocation are commenced by filing a request in writing at the EPO together with payment of the appropriate fee. As of March 2017, the fee for requesting central limitation is € 1,165 and the fee for requesting central revocation is € 525.
In the case of both limitation and revocation, the request must indicate the patent number, the patent proprietor and the contracting states in which the patent is in force (Rule 92(2) EPC). If the patent is owned by different proprietors in different contracting states, the requesting party must provide evidence that they are authorised to act on behalf of all proprietors (Rule 92(2)(c) EPC). The requestor must include the amended claims, together with amended description pages where appropriate, if central limitation is sought. There is no requirement to give the reason for requesting central limitation or revocation (Guidelines D-X 4.2). In particular, when filing a request for central limitation, there is no requirement to identify the prior art which the restricted claims are intended to distinguish from.
6. How does the EPO examine a request for central limitation or revocation?
In accordance with Rule 91 EPC, the Examining Divisions of the EPO are responsible for processing and deciding upon requests for central limitation and revocation. The first stage in both the central limitation and revocation procedures is an initial examination to ensure that the request meets the basic formal requirements outlined in the previous section. The EPO will contact the requestor if the request is formally deficient, and will set a time limit in which any deficiencies must be rectified. Failure to rectify the deficiencies within the time limit will result in the request being deemed inadmissible. The Examining Division will also check that no opposition proceedings are pending, since opposition proceedings supersede both central limitation and revocation proceedings. If opposition proceedings are pending, then the request will be rejected and the limitation fee refunded.
Once the Examining Division is satisfied that the request is admissible, it will begin the second stage of the procedure. For central revocation, this entails the EPO informing the requestor that their request is allowed. At the same time, the Examining Division indicates the date when the revocation decision will be published (Rule 95(1) EPC).
The second stage of the central limitation procedure involves a review of the proposed amendments. They are firstly examined to check that they represent a limitation of the existing claims, which means that the scope of at least one independent or dependent claim has been restricted. It should be borne in mind that this is not necessarily assessed with respect to the claims as granted, as the claims might already have been limited through a previous central limitation procedure. As central limitation has retroactive effect, claims that have been limited by a previous central limitation procedure become the granted claims and are therefore the claims against which ‘limitation’ is assessed.
The amendments proposed by the requestor are also examined to ensure that they comply with the clarity, sufficiency and added subject-matter provisions of the European Patent Convention. It will also be checked that the amendments do not result in the broadening of the patent’s scope in any way. It is necessary to explain where basis is provided in the application as originally filed for the limited claims when filing a request for limitation in order to show that the limited claims do not add subject-matter. The Examiner will not assess substantive patentability requirements, such as novelty and inventive step when examining a request for limitation.
If the Examiner does not consider the limitation request to be allowable during this second stage of the procedure, for example if the claims are considered to add subject-matter, a Communication will be issued inviting the requestor to address the objections raised. Failure to overcome the objections at this first attempt may result in the request for limitation being rejected (Rule 95(4) EPC). The requestor is only entitled by right to one opportunity to address objections which are raised. The EPO will occasionally give the requestor an opportunity to address any remaining objections, but this is generally only if such objections have arisen as a result of the requestor’s response to the first Communication. Since the requestor is afforded limited opportunities to address objections raised by the Examiner, it is prudent to include a precautionary request for oral proceedings, either when filing the limitation request or when responding to a Communication raising objections against the proposed claims.
The examination of limitation requests is an ex parte procedure, meaning that the request for limitation cannot be contested by a third party in the same way as validity can be contested in opposition proceedings. Third parties can, however, file observations in order to draw the attention of the Examining Division to reasons why the request for limitation should not be allowed. As with third party observations filed in other EPO proceedings, such observations can be filed anonymously.
7. Obtaining Different Claims for Separate Contracting States
The EPC permits a proprietor to file different sets of claims for different contracting states if the proprietor can show that this is appropriate due to the existence of national prior rights, which would be relevant to the validity of the claims in one or more designated contracting states (Rule 138 EPC). The central limitation procedure can be used to request different limitations to the claims for different contracting states.
If the patent proprietor requests the EPO to limit the claims for only a limited selection of contracting states, or wishes to request different limitations for different contracting states, then this has to be explained when making a request for central limitation. In this case, it is necessary to file a copy of the relevant national prior right(s) pursuant to Rule 138 EPC to evidence that different claims for different states are warranted.
8. Successful Request
If a request for revocation meets the formal requirements previously outlined, then the requestor is informed of both the positive outcome and the date upon which the revocation will be published by the EPO (Rule 95(1) EPC).
For successful limitation requests, however, there is an additional stage before the outcome is published by the EPO. This additional stage allows the requestor to check the documents which the EPO intends to publish as the limited patent (Rule 95(3) EPC). It is permissible to correct simple typographic errors at this stage, but it is not permissible to make substantive amendments to the text. It is also necessary to pay a publication fee and to file translations of the claims into the two official languages of the EPO that are not the language of the proceedings.
9. Unsuccessful Request
An unsuccessful request for limitation can be appealed. Appeals against adverse central limitation decisions at the EPO are processed by a Technical Board of Appeal. This is a relatively expensive procedure, and currently very slow due to a significant backlog of cases pending with the Appeal Boards. A more practical approach would be to file a new limitation request which cures, as far as possible, the deficiencies identified by the Examining Division in the earlier refused request.
PART D National and EU Law Related to EPC
I. EPC and National Law
- Rights Conferred by European Patents
- Validation and Translation Requirement
- The Prohibition of Double Patenting
- Annuities
- National Jurisdiction and Applicability of EPC and EP Case Law
- Precedence of EPO Opposition Proceedings Over National Invalidation Proceedings
1. Rights Conferred by European Patents
As of the date of grant, a European patent shall confer on its proprietor in each contracting state in respect of which it is granted the same rights as would be conferred by a national patent granted in that state (Article 64(1) EPC). Moreover, the national law must comply with Article 64(2) EPC stipulating that, if the subject- matter of the European patent is a process, the protection conferred by the patent shall extend to the products directly obtained by such process.
This provision is of great practical significance.
Example: A European patent application describes a new and inventive catalyst for making polyethylene and proceeds to grant on the basis of claims which exclusively concern the catalyst as such. If a competitor of the proprietor uses this catalyst in the United States for the preparation of polyethylene and delivers this polyethylene to an EPC contracting state, these actions do not infringe the patent.
However, the addition of process claims or use claims prior to grant could provide a suitable basis for asserting an infringement of the patent. A proper use claim would for instance read:
Use of catalyst X for making polyethylene.
A suitable process claim would be:
Process for the manufacture of polyethylene comprising the step of polymerizing ethylene in the presence of catalyst X.
Since the polyethylene delivered to Europe is the product directly obtained by such use (process), Article 64(2) EPC thus provides valuable protection.
However, the EPC does not contain any provisions specifying what actions constitute an infringement, how an infringement can be asserted and what claims result therefrom if the infringer is held to be liable by a national court. The EPC only states that any infringement of a European patent shall be dealt with by national law (Article 64(3) EPC).
2. Validation and Translation Requirement
The European patent does not represent a unitary patent. Once the decision to grant a European patent takes effect, i.e. on the date the grant is published in the European Patent Bulletin (Article 97(3) EPC) it falls apart into a bundle of national patent rights. Each of these national patent rights is subject to the respective national jurisdiction. There are, however, two important exceptions to this principle where the EPO retains its jurisdiction over the European patent as a whole, namely opposition proceedings pursuant to Article 99 EPC (see chapter 3.1) and the central request for limitation or revocation pursuant to Article 105(a) EPC).
In some EPC contracting states such as the United Kingdom (UK), France (FR), or Germany (DE), no further steps are to be taken after grant in order to validate the corresponding national right. In these countries, the timely payment of national annuities which have fallen due will suffice to maintain the national right until it expires 20 years from the application date. In other contracting states national rights will only come into existence if certain legal requirements are met. These typically include the submission of a translation of the claims and if applicable also of the description into the official language of the respective state as well as the appointment of a national representative or at least the indication of an address of service.
Any contracting state may, if the European patent, as granted, amended (after opposition proceedings) or limited by the EPO, is not drawn up in one of its official languages, prescribe that the proprietor of the patent shall supply to its central industrial property office a translation of the patent in one of the official languages. The period for supplying the translation shall end three months after the date on which the mention of grant, maintenance in amended form or limitation of the European patent is published in the European Patent Bulletin (Article 65(1) EPC). Currently, only two states make use of the possibility provided in Article 65(1) EPC to prescribe longer periods, namely San Marino (6 months) and Iceland (4 months). If the translation of the European patent is not provided to the national patent office within the prescribed time limit, the patent is deemed to be void ab initio in that state.
The EPO publishes and updates on its website a summary of applicable national provisions (https://www.epo.org/law-practice/legal-texts/national-law.html). Furthermore, the EPO issues at regular intervals a booklet entitled “National Law Relating to the EPC” with which the EPO aims to provide European patent applicants and proprietors with a concise guide to the most important provisions and requirements of the relevant national law in the EPC contracting states.
From these publications it can be gathered which measures, if any, are necessary to validate a European patent in the respective state and the applicable terms.
For many years, the translation requirement had been the main reason for the very high costs associated with a European patent and the main obstacle for patent proprietors to validate a European patent in a greater number of states. Aiming at reducing the translation costs, several EPC contracting states concluded in London on October 17, 2000 the “Agreement on the Application of Article 65 of the Convention on the Grant of European Patents”, frequently referred to as the “London Agreement”. The London Agreement entered into force for 14 states on May 1, 2008 and after the accession of further EPC contracting states, is now valid in 21 states. The Agreement provides that contracting states that have an official language in common with an official language of the EPO, i.e. English, French or German, no longer require translations of European patents into one of their official languages. Other contracting states have to choose one of the official EPO languages as a “prescribed language”, into which European patents have to be translated in order to enter into force in their country. They, however, keep the right to require a translation of the claims in one of their official languages.
The current implementation of the London Agreement is shown in the following table.

1) These states dispense with further translation requirements if the European patent has been granted in English, or translated into English and supplied under the conditions provided for in Article 65(1) EPC (Article 1(2) of the London Agreement). In Denmark, Finland, Hungary, Iceland, the Netherlands, Norway and Sweden, the European patent may also be supplied in the national language.
2) These states dispense with translation requirements for the description (Article 1(2) of the London Agreement).
3) Belgium dispenses with the translation requirements under Article 65(1) EPC for European patents that have been granted in English on or after January 1, 2017. As hitherto, for the validation of European patents granted in French or German (which are Belgian official languages) no translation needs to be filed. A ratification of the London Agreement can be expected in the near future.
4) If not stated otherwise, a translation into the official language of the state concerned is required
The EPO does not publish any statistics showing to what extent EP patents are validated in the individual contracting states. Straathof and van Veldhuizen investigated in 2010 the average validation rates for the EPC contracting state in 2005 (http://www.voxeu.org/article/another-reason-eu-patent-declining- validation-rates). These essentially correlate with the share of this state in the gross domestic product (GDP) of all EPC contracting states. Thus, not surprisingly, the likelihood of validation in a particular state increases with its economic relevance. This explains why (in 2005) 92% of all European patents were validated in Germany and about 80% in the United Kingdom and France, while the validation rates for Italy and Spain dropped already to about 50% and 33%, respectively. This is shown in the following graph:

Straathof, van Veldhuizen also observed that in the period from 1985 to 2008 smaller countries experienced a substantial decrease in validation shares, while the shares of large economies have remained approximately constant. Whether the London agreement has stopped or reversed this trend is currently unknown.
3. The Prohibition of Double Patenting
It is an accepted principle in most patent systems that two patents cannot be grantedtothesame applicantforoneinvention. The EPC leaves ittothecontracting state to permit or prohibit simultaneous protection of the same invention by means of a national patent and the national part of a European patent. According to Article 139(3) EPC, any contracting state may prescribe whether and on what terms an invention disclosed in both a European patent application or patent and a national application or patent having the same date of filing or, where priority is claimed, the same date of priority, may be protected simultaneously by both applications/patents.
Most EPC contracting states have made use of this possibility and enacted laws prohibiting double protection. However, double protection is permitted in AT, DK, FI, HU, IS, NO, PL and SE. If double protection is not allowed, the national patent loses its effect to the extent to which the scope of the claims overlaps with the scope of the claims of the EP patent.
In some countries the national patent ceases (at least partially) to have effect from the date on which
(a) the period for filing the notice of opposition to the European patent has expired without such notice being filed; or
(b) the opposition proceedings are finally closed, the European patent having been maintained.
In the United Kingdom the Comptroller may revoke the national patent after the dates provided in a) or b).
In a further group of countries, including France, Germany, Spain and Italy, the prohibition of double protection also applies
(c) if the national patent is granted if such date falls after that provided for in (a) or (b).
Then, the national patent will not take effect if its scope fully overlaps with that of the corresponding EP patent, or only in part if its scope does not fully overlap.
If hence a European patent application claims the priority of a national patent application deposited in an EPC contracting state, applicants should carefully examine whether double patenting issues can arise and, if so, what measures are to be taken in order to prevent a national patent from (partially) losing its effect. If this is undesired, it is as a rule recommendable to withdraw the designation of a corresponding EPC contracting state prior to grant.
4. Annuities
The national patent offices may only impose renewal fees for a European patent for the years which follow the year in which the mention of the grant of the European patent is published in the European Patent Bulletin (Articles 141 (1) and 86(2) EPC).
Example: The European Patent Bulletin publishes on June 3, 2011 the mention of grant of a European patent application having June 2, 2008 as filing date. The renewal fee for the third year (due on June 30, 2010) and the renewal fee for the fourth year (due on June 30, 2011) are to be paid at the EPO. The renewal fee for the fifth and all following years are to be paid at the national patent offices.
In this example, the fourth patent year has already commenced to run on June 3, 2011. Therefore, the mention of grant is published in the fourth patent year with the consequence that the renewal fee for the fourth year is still to be paid at the EPO.
The renewal fees to be paid to the national offices are much lower than those fixed by the EPO. Even if the total amount of renewal fees incurring at the national offices of those contracting states (DE, UK, FR and IT) having the highest validation rates is considered, there is still a substantial gap to the amount charged by the EPO as can be seen from the following graph:

5. National Jurisdiction and Applicability of EPC and EP Case Law
As of grant, in each state where the European patent was validated, the corresponding national part is subject to the jurisdiction of the national courts with the exception discussed below in Section 6. The EPC prescribes the national courts in Article 138, which grounds may be relied on to revoke a European patent with effect for a contracting state:
(a) the subject-matter of the European patent is not patentable under Articles 52 to 57;
(b) the European patent does not disclose the invention in a manner sufficiently clear and complete for it to be carried out by a person skilled in the art;
(c) the subject-matter of the European patent extends beyond the content of the application as filed or, if the patent was granted on a divisional application or on a new application filed under Article 61, beyond the content of the earlier application as filed;
(d) the protection conferred by the European patent has been extended; or
(e) the proprietor of the European patent is not entitled under Article 60, paragraph 1.
Item (a) includes the common revocation grounds of lacking novelty, lack of inventive step and exclusion from patentability. Items (a), (b) and (c) hence define the same grounds as can be invoked under Article 100 EPC when lodging an opposition to a European patent.
Grounds (d) and (e), however, are only applicable in national revocation proceedings. Ground (d) applies if, contrary to Article 123(3) EPC, the protection conferred by the European patent has been extended due to amendments conducted in opposition or limitation proceedings. For questions of entitlement, as referred to in ground (e), the EPO is also not competent.
The national law and practice of the EPC contracting states ensures the full applicability of Article 138. Pursuant to Article 138(2)(3), the proprietor of the patent shall also have the right to limit the patent by amending the claims if the grounds for revocation affect the European patent only in part.
In view of the link to the national law provided in Article 138, the practically very significant question arises of whether, and to what extent, the national courts follow the case law of the EPO Boards of Appeal when examining a patent in respect of the invoked revocation grounds.
The interpretation of the EPC by the Legal and Technical Boards of the Appeal and the Enlarged Board of Appeal is not binding for the national courts. This had the consequence that, after the entry into force of the EPC on October 7, 1977, the national courts continued their practice for many years with only few adaptations to the practice of the EPO. The resulting discrepancies between national decisions concerning the same European patent were perceived by users as a major drawback of the European patent system. Only in the last decade have the national courts, especially the judges in the UK, Germany and the Netherlands, endeavoured for a greater harmonization with the EPO practice.
The German Federal Court of Justice highlights for instance in Walzenformgebungsmaschine (OJ EPO 11/2010, 622) that:
the German courts are required to consider decisions rendered by organs of the European Patent Office and courts in other EPC contracting states and pertaining to a largely similar issue and, where appropriate, address the reasons leading to a diverging result in the earlier decision. Insofar as points of law are concerned, this also applies, for instance, to the question of whether the subject-matter of a property right was obvious in the light of prior art.
The Federal Court of Justice emphasizes that this approach is necessary both from the point of view of legal certainty as well as in the interest of harmonizing the case law in EPC contracting states.
Likewise, the House of Lords, which is the highest court in the UK, held in Kirin- Amgen Inc v Hoechst Marion Roussel Ltd ([2004] UKHL 46) that:
the United Kingdom should apply the same law as the EPO and the other Member States when deciding what counts as new for the purposes of the EPC.
Although the decisions of the Boards of Appeal of the EPO are not binding on the UK courts, these decisions have been described by the House of Lords as being of “great persuasive authority” both because they are from “expert courts”, and furthermore because “it would be highly undesirable for the provisions of the EPC to be construed differently in the EPO from the way they are interpreted in the national courts of a Contracting State”.
To some more limited extent, French courts usually take the reasoning of the EPO decisions into account when deciding on the validity of a patent. In this regard, the Paris Court of First Instance held in Actavis v. Merck (TGI, 3rd ch. 1st sect., September 28, 2010) that:
The French courts are not bound by the decisions of the EPO which is not a court so that these decisions even issued by the Enlarged Board of Appeal are merely indications of the analysis made by the EPO to grant European patents.
Despite the trend towards harmonization, major differences continue to exist between the respective national practice of the EPC contracting states and that of the EPO. The national courts in the majority of EPC contracting states including the German and British courts seem to be more lenient regarding the allowability of amendments and reject the strict added matter test developed by the EPO, which often seems to require a literal disclosure of the desired amendment.
When judging the admissibility of amendments in light of the disclosure of the application as filed, the German Federal Court of Justice has repeatedly held, e.g. in “Spleißkammer” (X ZB 9/89 of January 23, 1989) and “Kommunikationskanal” (X ZR 107/12 of February 11, 2014) that it would generally not be inadmissible to incorporate only some of a plurality of features of an embodiment into the independent claims. If features of an embodiment either jointly or individually contribute to the success achieved with the invention, and serve to specify the protected invention, it is essentially permissible that the patent be limited by incorporating such features, individually or in their entirety, into the claim.
If, in proceedings before the EPO, an amendment involves a singling-out of features from a combination of features disclosed in one working example, the yardstick for the admissibility of the amendment is however whether the skilled person could recognize without any doubt that those (singled-out) features/ characteristics were not so closely related to the other features/characteristics of the working example and that they apply directly and unambiguously to the more general context (see e.g. T962/98).
Meanwhile, in a recent UK case, the Court of Appeal held that the generalization of the description of a part of a brake calliper as “hockey-stick shaped” in the application as filed to“asymmetric shaped”in the granted patent was an allowable amendment (AP Racing Ltd v Alcon Components Ltd [2014] EWCA Civ 40). This seems to be more lenient than the approach of the Boards of Appeal of the EPO. For example, in T 653/03, it was held that the replacement of the specific term “diesel engine” disclosed in the application as filed with the undisclosed general term “combustion engine” was not allowable.
Another practically important area where the national courts do not, or do not entirely, follow the EPO is the assessment of inventive step, and especially the application of the problem-solution approach developed by the EPO.
The first step of the problem-solution approach involves the determination of the so-called “closest prior art”. By contrast, according to the jurisprudence of the German Federal Court of Justice (BGH GRUR 2009, 1039 – Fischbissanzeiger), the choice of a specific starting point requires a particular justification. Accordingly, inventive step is typically assessed by the German courts in light of several documents as possible starting points. On the other hand, the Federal Court of Justice emphasized in a number of recent decisions that, in order to conclude lack of inventive step, it is insufficient for the nullity plaintiff to show that the technical problem was recognizable. Beyond this, the prior art must furnish an inducement, motivation, hint or other cause to seek the solution of the technical problem in the same way as the invention (Betrieb einer Sicherheitseinrichtung, BGH GRUR 2009, 746). Since this test links the question of obviousness with the technical success accomplished by the invention, it will frequently lead to similar results as the problem-solution approach of the EPO. At the same time, the inventive step assessment by the German courts does not only rely on an objectively defined problem, but considers equally other reasons which may induce a skilled person to modify the existing art, such as simplification, saving of costs and energy reduction or the like. These practically important causes for technical developments are often not considered by the problem-solution approach and can have the consequence that a European patent is declared null and void in Germany even if it was previously held to be valid by the EPO.
The practice of the British courts is even further apart from that of the EPO. A set of rules regarding the approach taken by the United Kingdom courts was laid out by the Court of Appeal in Windsurfing International Inc. v Tabur Marine (GB) Ltd. [1985] RPC 59, in determining the requirements for inventive step. This test has been slightly reworked in the more recent Court of Appeal case Pozzoli Spa v BDMO SA & Anor [2007] EWCA Civ 588 (June 22, 2007) as follows:
- (a) Identify the notional “person skilled in the art”, (b) Identify the relevant common general knowledge of that person;
- Identify the inventive concept of the claim in question or if that cannot readily be done, construe it;
- Identify what, if any, differences exist between the matter cited as forming part of the “state of the art” and the inventive concept of the claim or the claim as construed;
- Viewed without any knowledge of the alleged invention as claimed, do those differences constitute steps which would have been obvious to the person skilled in the art or do they require any degree of invention?
It follows from this approach that, in contrast to the problem-solution approach applied by the EPO, the UK practice does not involve a mosaicing of documents (combination of prior art teachings). The information and teaching provided by secondary documents is rather considered in step 1(b), where the common general knowledge of the person skilled in the art is identified. It is easily understood that this approach can lead to decisions diverging from those rendered by the EPO
6. Precedence of EPO Opposition Proceedings Over National Invalidation Proceedings
The national jurisdiction as of grant of a European patent does not necessarily mean that the respective national part can be immediately attacked in invalidation proceedings. In order to avoid diverging decisions and to relieve the national courts, several EPC states have stipulated that EPO opposition proceedings take precedence over national invalidation proceedings. According to section 81(2) of the German Patent Act, an action for declaration of nullity of a patent may not be brought as long as an opposition may still be filed or opposition proceedings are pending.
Similar provisions are found in the national laws of France.
On the contrary, in Italy and Spain there is no provision that prevents the starting of an action for declaration of nullity of a patent in the period during which an opposition may be filed or opposition proceedings are pending.
Another notable exception is the UK, where the courts assess whether or not to stay proceedings in line with the principles set out by the Court of Appeal in IPcom v HTC Europe ([2013] EWCA Civ 1496). In this decision, the Court of Appeal held that a stay of national proceedings is the default option, but can be refused at the discretion of the court. Some of the factors considered in reaching this decision are:
- the length of time that it will take for the proceedings to reach a conclusion,
- whether there is evidence that commercial certainty would be achieved at an earlier date in UK proceedings, and
- whether the refusal of a stay will irrevocably deprive a party of a benefit which would be available if the proceedings were stayed.
- Also in the Netherlands it is at the discretion of the judges as to whether nullity proceedings are stayed or not.
II. The European Patent within the future EU Patent Package
- Introduction
- Legal Basis
- How to Obtain an EP-UE
- The Territorial Scope of an EP-UEs
- Unitary Effect
- Translations
- Renewal Fees
- For whom will an EP-UE commercially make sense?
- Start of the EP-UE and the Implications of Brexit
1. Introduction
At the date of finishing this article (March 2017), it seems likely that a new type of patent, the so-called European Patent with Unitary Effect, will come into existence in the not too distant future, even though it is yet unclear when exactly this will happen, as it depends on a number of political developments which are in flux and will briefly be discussed at the end of this chapter. In this chapter, we will be using the abbreviation EP-UE since it indicates that the EP-UE is based on a granted European patent (EP). Just like any other European patent, the EP-UE is filed at, prosecuted, and eventually granted by the European Patent Office (EPO).
2. Legal Basis
At the end of 2012, agreement was reached on the legal instruments constituting the so-called EU Patent Package. These legal instruments are two EU Regulations [13]creating the European Patent with Unitary Effect (EP-UE) and setting forth the translation requirements, respectively, and the Unified Patent Court Agreement (UPCA) [14] as an international agreement. The EP-UE Regulations make use of an option provided in the European Patent Convention to validate European patents supra-nationally.
3. How to Obtain an EP-UE
How can a European Patent with Unitary Effect (EP-UE) be obtained? Basically by the following three steps:
- applying and prosecuting to grant a European patent at the European Patent Office (EPO), designating all member states participating in the EU patent package;
- requesting the EPO to have the European patent registered as an EP-UE within one month after grant of the European patent; and
- submitting a translation of the entire patent together with the request for registration as an EP-UE, i.e. apparently also within the above one- month time limit, for a transitional period of at least six, and possibly up to twelve years.
From the date the EP-UE Regulation becomes applicable, i.e. the date of entering into force of the UPCA, any European patent which at that time is still within the one-month period after grant can be registered as an EP-UE. This means, EP-UEs can be registered from day one since they are prosecuted as “normal” European patents.
4. The Territorial Scope of an EP-UE
The territorial scope of the EP-UE will be determined by the EU Member States’
(a) participation in the enhanced cooperation to create the EP-UE Regulation and
(b) ratification of the UPCA. Here we will be referring to countries fulfilling both conditions as EP-UE Member States. Whether the UK will eventually also become and/or remain an EP-UE Member State following the Brexit decision, is still unclear (see last section of this chapter) but it will in any case remain a member of the European Patent Organisation (EPO), so that classic EP bundle patents will continue to be available for the UK.
The request for enhanced cooperation (a) has been signed by 25 countries so far (all EU Member States except Croatia and Spain).
The requirements for ratifying the UPCA (b) differ considerably withinthe signatory states. Therefore it can be expected that if and when the UPCA enters into force, the initial territory where the UPCA enters into force will not be constituted by all states, and some states will be subsequently joining from time-to-time.
For example, Spain and Poland have indicated that they will not join the agreement in the foreseeable future. The Czech republic has announced that it will wait with the ratification until really reliable machine translations into Czech will be available, and Croatia has, to date, neither signed the UPCA nor indicated that it will join the enhanced co-operation for establishing the EP-UE. Also CY, GR, and SK seem to want to stay out for now, and whether there is a future for GB as a UPCA member state is quite unclear.
A granted European patent having been registered as an EP-UE will have effect in all of those countries where the UPCA had entered into force at the time of registration as an EP-UE. Let us assume the UPCA initially enters into force only in 13 states: a European patent which is registered immediately thereafter will cover only the territory of those 13 states. The territorial scope of an EP-UE once registered will also not be subsequently extended when the UPCA is ratified in further states. Only European patents that are later registered as EP-UEs, i.e. after the UPCA has entered into force in the further states, will enjoy the enlarged territory.
For all EU Member States which either did not participate in the enhanced cooperation or where the UPCA has not yet entered into force (Non-EP-UE Member States), the European patent will still have to be validated nationally as before. The same will apply permanently with regard to all EPC Member States that are not EU Member States, such as Turkey, Switzerland, Norway. As regards the UK, see the last section of this chapter.
National validation will therefore remain available to complement the registration as EP-UE. This means that applicants will have a choice in the future for many EU member states whether they want an EP-UE or rather a classic European bundle patent [15].
The determination of the territorial scope as of the time of registration as an EP-UE must lead to two considerations. First, in some cases it might be advantageous to delay the grant of a European patent to ensure a broader territorial scope. Should it be foreseeable when the Examining Division gives notice of its intention to grant the European patent that the UPCA will soon enter into force in further relevant EU Member States, the patentee may save renewal fees and possibly translation costs if it delays the grant so as to ensure that when the European patent is registered as an EP-UE, the UPCA will already have entered into force in those countries. Secondly, the time when the registration as an EP-UE is effected will not be in the hands of the patentee. It will depend on the speed of the registration process. If a country where the patent shall have effect is about to join the EP- UE territory (become a EP-UE Member State), but it is not clear whether this will happen before or after the patent’s registration as an EP-UE, the patentee may have to initiate a national validation as a safety measure. This again may speak in favor of delaying the grant if a desired country is about to become an EP-UE Member Stat.
5. Unitary Effect
With the supra-national validation of a European patent as an EP-UE by registration at the EPO, the EP-UE will become a “unitary right”. Upon registration of unitary effect, the EP-UE shall have unitary character retroactively as of the date of publication of mention of grant in the EPO Official Bulletin in all EP-UE Member States at the time of registration. Unlike nationally validated European patents, EP-UEs will have the same legal fate in all covered EP-UE Member States. The EP- UE can only be invalidated, limited or allowed to lapse with regard to its entire territory. It can also be assigned only for its entire territory. Licenses can, however, be limited to certain territories.
For patentees, one advantage of this unitary effect will be that renewal fees will have to be paid only at one patent office, the EPO, and not at each national patent office. On the other hand, the practice of letting less important national EP parts later lapse to reduce renewal fees will not be available for the EP-UE. Also, with regard to their particularly valuable patents, patentees may dislike the simplified invalidation by a central action before the Unified Patent Court (UPC) instead of national invalidation proceedings in all relevant countries, as is currently the case with European patents after expiry of the nine-month opposition period. National invalidation is cumbersome and expensive for a competitor and bears the risk that the patent will remain in force at least in some countries.
A further aspect is the applicable property law. Property laws are relevant for a number of questions such as the rights of co-owners as well as the requirements for assigning, licensing, and providing a patent as security. The European Patent Convention (EPC) does not set forth comprehensive provisions on the EP as a property. Instead it refers to the property laws of the countries in which the patent will have effect after grant. Upon grant, a European patent is treated like a national patent such that the respective national property laws apply to each of its national parts. However, since the EP-UE is a unitary right, this solution of applying a different property law to each of its national parts could not be maintained for the EP-UE. The drafters of the EU Patent Package had to find a different solution which sets forth one specific property law being applicable to the entire unitary right. They resolved this not by creating a specific property law only for EP-UEs, but by setting forth conflict-of-law rules which select the national property law that is applicable to an EP-UE. According to these conflict-of-law rules, simply put, the property law of the EP-UE Member State shall apply where the applicant had its residence or its (principal) place of business at the filing date of the European patent application. Should the applicant have no residence or place of business in any EP-UE Member State, German property law shall apply as default. For the many applicants from Asia and the USA, German property law will therefore become more relevant.
6. Translations
To register an EP-UE, a translation of the entire patent will be required during a transitional period of at least six, and at most 12 years. The transitional period shall end when an independent committee has established that machine translations into all official languages of the EU Member States have attained a sufficiently good quality. The EPO is working to improve such translations in cooperation with Google.
During the transitional period, the following provisions apply: (1) If the European patent was prosecuted and granted in German or French, an English translation will be required. (2) If the European patent was prosecuted in English (which is predominantly the case – about 80% of EP’s are in English), the applicants will have to provide a translation into any other official language of the European Union. This even includes the languages of those EU Member States which the EP-UE will not cover – at least in the foreseeable future -, such as Spanish, or Polish.
The decision as to which language to choose for the translation will mainly be a commercial one. If the European patent is to be additionally validated also in Spain or Poland, a national validation in these countries will require a translation of the patent into the official languages of these countries anyway. Such a translation could then also be used for registering the EP-UE.
The correctness of the EP-UE translation does not have legal relevance; it is for information purposes only. This is different from the translations required for some national validations.
Moreover, a translation of the EP-UE may be required when enforcing it, namely if the proceedings are conducted in a language other than the language of the granted patent and the submitted translation. This will apply also beyond the transition period.
7. Renewal Fees
The renewal fees for the future EP-UE have meanwhile been determined and will be approximately equivalent to the cumulated renewal fees of the TOP4 states (DE, FR, GB, NL).
It is thus clear that applicants who previously only validated their European patents in the three most important member states (DE, GB, FR) will have to pay significantly more than that now when they want to acquire an EP-UE. This applies to about 50% of all applicants. A further 40% of the applicants have so far validated their European patents in DE, FR, GB, IT and ES. They will also likely experience no savings in renewal fees, since at least for Spain a national validation will still be necessary and the NL fees that go into the EP-UE renewal fees are much higher than the IT renewal fees used to be. Another disadvantage of the EP-UE is that it is not possible to drop individual designations as in the past. On the other hand, the territory of the EP-UE will be much larger than the combined territory of DE, GB, FR and NL.
The sum of the total renewal fees over 20 years is expected to be 35,555 EUR.
8. For whom will an EP-UE commercially make sense?
Back to the table of contents
Patentees faced with the decision of whether to register their European patents as EP-UEs will have to consider the advantages and disadvantages. Unfortunately, this is quite a complex assessment, even more so following the “Brexit” decision by the UK referendum, and many factors can play a role. The following considerations cannot therefore be comprehensive, but they should provide the reader with some initial ideas.
8.1 Cost of an EP-UE Compared to National Validation
If one were to compare the costs of an EP-UE to the costs of validating a patent nationally in all EP-UE Member States, the EP-UE would certainly be more cost efficient. However, this is an overly simplistic view since in practice European patents are validated only in selected countries and most patentees are under such budget constraints that they will mainly consider how the EP-UE compares to their established validation strategy. For such a comparison, the following may be considered:
8.2 Renewal Fees
The renewal fees for the EP-UE will be at around the TOP4 level (see above). Whether this is more or less than the costs of a national validation will depend on the countries where the European patent would otherwise be validated. For example, the accumulated renewal fees of the new EP-UE until the end of its 20- year lifetime will be about 10,000€ higher than the accumulated renewal fees for DE, FR and GB. However, the average lifetime of an EP is currently only about 11 years, so that the disadvantage will be much less pronounced.
A further factor to be considered are the savings which can be achieved in administrative costs and fees for paying multiple renewal fees versus one renewal fee for an EP-UE.
8.3 Translation Costs
At least in the transitional period in which the request for a registration as an EP-UE must be submitted together with a translation of the European patent, translation costs will also remain relevant. To compare the translation costs for a complete translation of a patent for EP-UE registration with the translation costs otherwise incurred for national validations, even more factors must be taken into account. The translation costs will also depend on the number of pages to be translated and the differing costs depending on the language.
Again, for patentees who otherwise would only validate in the TOP3 states, i.e. Germany, the UK, and France, the translation costs incurred for an EP-UE will certainly be higher than for national validations. In all these countries no translation is required for national validation. If the patent is, however, to be validated also in Spain, the translation costs for an EP-UE will be identical to those for a EP patent plus a complete national validation. To validate a European patent in Spain, so as to complement the registration as an EP-UE, a translation into Spanish will be required which can be used also for registering the EP-UE. If the validation pattern should be Germany, UK, France and The Netherlands, the translation costs for the EP-UE will again be higher than for national validations since for a national validation in The Netherlands only a translation of the claims is required. For the UK, no extra translation will be necessary, since the claims of an EP patent must be in English language or be translated into English anyway.
On the other hand, if a patentee wants to take advantage of the entire territorial scope of the EP-UE, this would infer a significant reduction in translation costs compared to the regular national validations.
8.4 Enforcement Costs
Enforcement costs may be regarded as being less important since only a small fraction of patents will be litigated. It should be noted, however, that the litigation costs will be generally higher at the newly established UPC than they are now in Germany, though probably lower than in the UK. This will, however, largely depend on the value of the matter in dispute. Thus, our statement is applicable for lower or “normal” values (up to about 3 million €), but not for very high values (such as 30 or 50 million €), as they are sometimes applicable in pharma or telecom cases.
8.5 The Other Side of the Equation: The Covered Territory
If a patentee should determine after considering the above that, compared to its established validation strategy, a EP-UE will be more expensive, it should also consider before abandoning the idea of registering an EP-UE that additional protection in further countries will be obtained. In which additional countries protection will be provided will depend on when the EP-UE is registered. As set out above, initially the EP-UE may only cover 13 or so EP-UE Member States. It will take a while until all 25 signatory states have ratified the UPCA.
A patentee should consider whether the additional territorial scope is worth the additional costs of the EP-UE and whether it can find the money in its budget to obtain the additional protective scope which it has not deemed necessary up to that point.
As can be seen in the figure shown below [16], the EP-UE will upon ratification in all countries that have signed the UPCA cover in addition to at least three of the five biggest markets (DE, FR, IT) a large number of comparatively small economies, many of which are in Eastern Europe. These countries are presently developing and thus may become more important in the future.

8.6 Further Factors
Further factors to be considered are that an EP-UE will simplify the internal and external administrative processes compared to registering several national patents, not only with regard to renewal fees, but also in cases of transfers, name changes, etc.
The other side of the coin is less flexibility. With a portfolio of nationally validated European patents, a patentee will have the possibility to flexibly decide from time to time to reduce the number of countries in which the patent is to be maintained, in the extreme to one country, if the patent is later regarded to be less important, but not entirely dispensable. An EP-UE can, however, only be maintained or allowed to lapse in its entirety.
8.7 Conclusion: EP-UE, European Patent or National Patent?
Which of the three options to obtain patent protection in the EP-UE Member States is the best will have to be a case-by-case decision. In view of the probably higher enforcement costs and uncertainty as to the Unified Patent Court’s (UPC’s) future practice, some applicants may at least initially avoid the EP-UE and instead stick with the existing EP bundle patent and opt out from the UPC, or they may even be looking more closely at national patents in addition to or in lieu of filing a European patent application. The UPC will eventually have jurisdiction not only for EP-UEs, but also for nationally validated European patents in UPCA member states. However, during a transitional period of at least seven years, it will be possible for patentees to “opt out” from the UPC. This means that national courts will remain solely competent for enforcement or invalidation actions.
In sectors where infringements are necessarily Europe-wide, excluding an important market like Germany is in many cases not commercially feasible for a competitor; thus a national German patent or an EP patent validated in just DE, GB or FR may be entirely sufficient. The German government has been trying to make national German applications more attractive by allowing the initial filing of applications in English or French. A translation will only have to be filed after twelve months, and not later than 15 months from the priority date. A request for examination will not have to be submitted until after seven years from the application date and this will allow postponement of payment of a considerable share of the costs.1459
9. Start of the EP-UE and the Implications of Brexit
Caveat: The following section deals with political and legal issues that are in flux and may well change within a few weeks or months. The status described here is the one of March 1, 2017.
When and how will the EU Patent Package enter into force? Technically, the two EU Regulations establishing the EP-UE and governing the translation requirements, respectively, are already in force, but they will apply only if and when the Unified Patent Court Agreement (UPCA) also enters into force. The UPCA was signed on February 19, 2013 by 25 EU Member States, but it must still be ratified according to the respective constitutional requirements of the signatory states. The UPCA will enter into force when 3 plus 10 countries ratify it, the three countries being Germany, the United Kingdom, and France, and the other ten being any of the further 22 signatory states. Currently, 11 states have ratified the UPCA (AT, FR, BE, SE, DK, LU, MT, NL, PT, FI and BG). The ratification of Italy (IT) is almost completed, and Slovenia (SI) may also be expected to ratify in due course. In Germany (DE), the ratification process is proceeding through Parliament, but Germany has indicated that it would like to be the last of the three mandatory ratification states, so that the UPC and hence the EP-UE can start with Germany’s ratification when everything is ready. More precisely, the UPCA will enter into force on the first day of the fourth month after the last of the mandatory ratification states (DE in this case) has deposited its instrument of ratification. Further EU member states are welcome to join the UPCA whenever they are ready; the UPCA will likewise apply for them on the first day of the fourth month after they have deposited their respective instruments of ratification.
Thus, the only significant roadblock to the entry into force of the UPCA is the ratification of the UK (GB). Following the outcome of the 2016 referendum concerning Great Britain’s membership in the EU, which will result in GB leaving the EU (so-called Brexit), many doubted that the UK would still be interested in ratifying the UPCA. This is before the backdrop that the Court of Justice of the European Union (CJEU) has opined in C-01/09 that the UPC must be a court within the institutional and judicial framework of the European Union and, hence, be part of the judicial system provided for in Article 19(1) of the EU Treaty. As a consequence, the UPC must be able to refer questions of law to the CJEU and has to accept the primacy of decisions by the CJEU. One of the most pressing political questions is whether the new UK government really wants to accept that and is ready to join the EU patent package now, given that the UK as a whole will leave the EU in a few years and given that the UK government has clearly and repeatedly indicated that it will no longer accept primacy of CJEU jurisprudence post Brexit. If the UK wanted to remain within the UPCA, it would therefore have to make an exception for patents to its clearly expressed intention to “take back control” from (inter alia) the CJEU. We will see whether this will actually happen.
Nevertheless and to the surprise of many, the representative of the UK has confirmed to the EU Competitiveness Council that the UK “is proceeding with preparations to ratify the Unified Patent Court Agreement”. Whether this means that the UK will actually ratify the UPCA in Spring, as some observers assume and as seems to be derivable from the heading of a website of the UK government [17], is still open at present. In a hearing before the Commons Select Committee, the new UK Minister for Intellectual Property literally [18]testified: “We have taken a decision to ratify, ehm to proceed with preparations to ratify the Patent Court Agreement…”. Whether there is a subtle distinction to be discerned here, will have to be awaited. In any case, the two Houses of Parliament and the Scottish Parliament will still have to discuss and confirm the ratification.
Interestingly, the Minister also said: “This is not an EU institution, the Unified Patent Court, and it is independent of our membership to the European Union”, while apparently dodging the fact that “the UPC must respect and apply Union [= EU] law, and, in collaboration with the Court of Justice of the European Union as guardian of Union law, ensure its correct application and uniform interpretation; the Unified Patent Court must in particular cooperate with the Court of Justice of the European Union in properly interpreting Union law by relying on the latter’s case law and by requesting preliminary rulings in accordance with Article 267 TFEU”, as one of the recitals of the UPCA stipulates.
Assuming that the UK and Germany will ratify the UPCA in spring 2017, the Provisional Application Phase (PAP) of the UPC will start in summer or early autumn. The PAP will mean that the organization as such will be established including the start of operation of the UPC’s formal governing bodies. It will also mean that judicial interviews can begin and appointments eventually confirmed. The start of the sunrise-period for the possibility to opt out European patents is now planned such that a minimum of 3 months will be provided for patent holders who wish to opt out their patents to do so before the Court becomes operational. The Agreement on the Unified Patent Court (UPCA) can then enter into force and the Court become operational. While the Prepartory Committee of the UPC is currently working under the assumption that the court will become operational in December 2017, we consider this is wishful thinking (for which the Preparatory Committee has an [understandable] reputation) and rather assume that the earliest possible start will be in 2018.
If it turns out that the UK will not ratify the UPCA and hence not join the “EU Patent Package”, the entry into force of the EU Patent Package will be significantly delayed, since several aspects of the European Patent Package will then have to be re-negotiated, including the seat of the central division of the UPC for chemistry and pharma cases. Nevertheless, the political will of the remaining EU Member States participating in the enhanced cooperation on patents to enact the UPCA still appears unbroken and we believe that the UPC will come some day, either with our without the UK.
Assuming again that the UK will ratify the UPCA and the UPC will start in 2017 or 2018, the question arises what will happen when the UK will leave the EU, which will most likely be by the end of March 2019 (assuming that the UK government submits its notification under Art. 50 EU Treaty by end of March 2017 as the UK Prime Minister announced). This is completely unclear and extremely difficult to predict at this moment, since it depends on many complex political and legal questions. Among them is the question whether the UK will accept the primacy of Union law [19] and the CJEU jurisprudence for the purposes of disputes governed by the UPCA, and whether the UK is interested to also stay in the enhanced cooperation for establishing the EP-UE. It is possible that the UK may want to stay in the UPCA (which the UK minister does not regard as an EU institution) but may not accept the EP-UE, which is governed by two EU Regulations. In any case, the political question of whether the UK will stay in the UPCA even post-Brexit will be part of the complex and difficult negotiations between the UK and the EU, which will start once the UK has submitted its notification under Art. 50 EU Treaty and the outcome of which cannot be predicted today.
Among the various legal issues is the question whether a non-EU Member State is per se excluded from participating in an enhanced cooperation under EU law and whether a non-EU Member State may be (or remain) a member of the UPCA. For example, Art. 1, 2nd paragraph of the UPCA reads: “The Unified Patent Court shall be a court common to the Contracting Member States and thus subject to the same obligations under Union law as any national court of the Contracting Member States.” This might well (but does not necessarily have to) be interpreted in an exclusive sense. Ironically, it is not at all impossible that this question may come before the CJEU some day.
In summary, the exact launch date of the UPCA and the the EP-UE is not yet clear, but realistically the earliest possible date is some time in 2018. The EP-UE and the UPCA will continue to exist also after the UK has left the EU in (presumably) 2019, but it is yet unclear and essentially unpredictable, whether and to what extent the UK will remain a part of it.
[1] OJ 2009, 456 – http://archive.epo.org/epo/pubs/oj009/08_09_09/08_4569.pdf
[2] The TRIPS Agreement dates from 1994, i.e. it is twenty years younger than the EPC. Its Art. 27(1) reads: Patentable Subject Matter 1. Subject to the provisions of paragraphs 2 and 3, patents shall be available for any inventions, whether products or processes, in all fields of technology, provided that they are new, involve an inventive step and are capable of industrial application.
[3] Art 52(2) EPC lists the following (non-limiting) examples of subject matter that is not considered an invention: discoveries, scientific theories and mathematical methods; aesthetic creations; schemes, rules and methods for performing mental acts, playing games or doing business, and programs for computers; presentations of information. A common denominator of these types of subject matter is their “non- technical“ nature. The EPO does not accept patentability of “business methods”.
[4] not excluded are: microbiological processes or the products thereof
[5] not excluded are: products, in particular substances or compositions, for use in any of these methods (Art. 54(4) and (5) EPC),
[6] Like Art. 53(a) EPC, Art. 27(2) TRIPS speaks of “ordre public or morality”. The TRIPS agreement exemplifies this further, “including to protect human, animal or plant life or health or to avoid serious prejudice to the environment. Art. 53(b) EPC resembles Art. 27(3)(b) TRIPS, notably, however, TRIPS does not speak of “varieties” but more broadly of plants and animals. Art. 53(c)EPC resembles Art. 27(3)(a) TRIPS, “for the treatment of the human or animal body” is phrased “for the treatment of humans or animals” in TRIPS.
[7] Art. 5(2) of Regulation EC 2100/94
[8] EU Dir. 98/44/EC, rec. 29
[9] G2/07, Reasons for the decision 6.4.2.3
[10] G2/07, Reasons for the decision 6.4.2.3
[11] G2/07, Reasons for the decision 6.4.2.3
[12] EPO OJ, November 2007, pages 594 – 600.
[13] Regulation (EU) No. 1257/2012 of December 17, 2012 implementing enhanced cooperation in the area of the creation of unitary patent protection (the EP-UE Regulation) and Regulation (EU) No. 1260/2012 of December 17, 2012 implementing enhanced cooperation in the area of the creation of unitary patent protection with regard to the applicable translation arrangements (the Translation Regulation).
[14] Agreement on a Unified Patent Court of February 19, 2013 (UPCA).
[15] If a European patent has been registered as an EP-UE, an additional national validation in any country within the territorial scope of the EP-UE will be ineffective.
[16] This figure and the last one were drawn before the UK Brexit referendum. For a discussion of the implications of Brexit, see the last section of this chapter.
[17] https://www.gov.uk/government/news/uk-signals-green-light-to-unified-patent-court-agreement
[18] http://www.parliament.uk/business/committees/committees-a-z/commons-select/science-and-technology-committee/news-parliament-2015/intellectural-property-ev4-16-17/
[19] as enshrined in the UPCA Recital: “RECALLING the primacy of Union law, which includes the TEU, the TFEU, the Charter of Fundamental Rights of the European Union, the general principles of Union law as developed by the Court of Justice of the European Union, and in particular the right to an effective remedy before a tribunal and a fair and public hearing within a reasonable time by an independent and impartial tribunal, the case law of the Court of Justice of the European Union and secondary Union law.”
Authors: Thorsten Bausch, Matthias Kindler, Andreas Stefferl, Michele Baccelli, Morten Garberg, Stephan Disser, Klemens Stratmann, Veit-Peter Frank, Declan Mulhern, James M. Ogle, Georg Siegert, Jan-Hendrik Spilgies, Leonard L. Werner-Jones, Christiane Stein-Dräger, David Lethem, Matthias Wolf, Martin Bachelin, Rainer E. Zangs, Joachim Renken
back
